by All Things Neonatal | Nov 10, 2016 | hypoglycemia, Neonatal, Neonatology, Uncategorized
I have probably received more requests for our glucose gel protocol than any other question since I started writing on this blog. Dextrose gel has been used more and more often for treatment of hypoglycemia such that it is now a key strategy in the management of low blood sugar in ours and many other institutions. If you are interested in the past analyses of the supporting trials they can be found in these posts; Glucose gel For Managing Hypoglycemia. Can We Afford Not To Use It? and Dextrose gel for hypoglycemia: Safe in the long run? As you can tell from these posts I am a fan of dextrose gel and eagerly await our own analysis of the impact of using gel on NICU admission rates for one!
But What If You Could Prevent Hypoglycemia Rather Than Treating It?
This is the question that the same group who has conducted the other trials sought to answer in their dose finding study entitled Prophylactic Oral Dextrose Gel for Newborn Babies at Risk of Neonatal Hypoglycaemia: A Randomised Controlled Dose-Finding Trial (the Pre-hPOD Study). I suppose it was only a matter of time that someone asked the question; “What if we prophylactically gave at risk babies dextrose gel? Could we prevent them from becoming hypoglycemic and reduce admissions and improve breastfeeding rates as has been seen with treatment of established hypoglycemia?” That is what they went out and did. The group selected at risk patients such as those born to mothers with any type of diabetes, late preterm infants, SGA and others typically classified as being at risk but who did not require NICU admission at 1 hour of age when treatment was provided. The primary outcome was hypoglcyemia (<2.6 mmol/L) in the first 48 hours. Secondary outcomes included NICU admissions, breastfeeding rates in hospital and after discharge as well as formula intake at various time points.
The study sought really to serve as a pilot whose goal was to determine when compared to placebo whether several different regimens could prevent development of hypoglycemia. The groups were (with the first dose in each case given at 1 hour of age):
- Single dose of 40% dextrose gel – 0.5 mL/kg
- Single dose of 40% dextrose gel – 1 ml/kg
- Four doses of 0.5 mL/kg given every three hours with breastfeeding
- A single dose of 1 mL/kg then 3 X 0.5 mL/kg given q3h before each breastfeed.
In total 412 patients were randomized into 8 different groups (4 treatment and 4 placebo).
As The Saying Goes, Less Is More
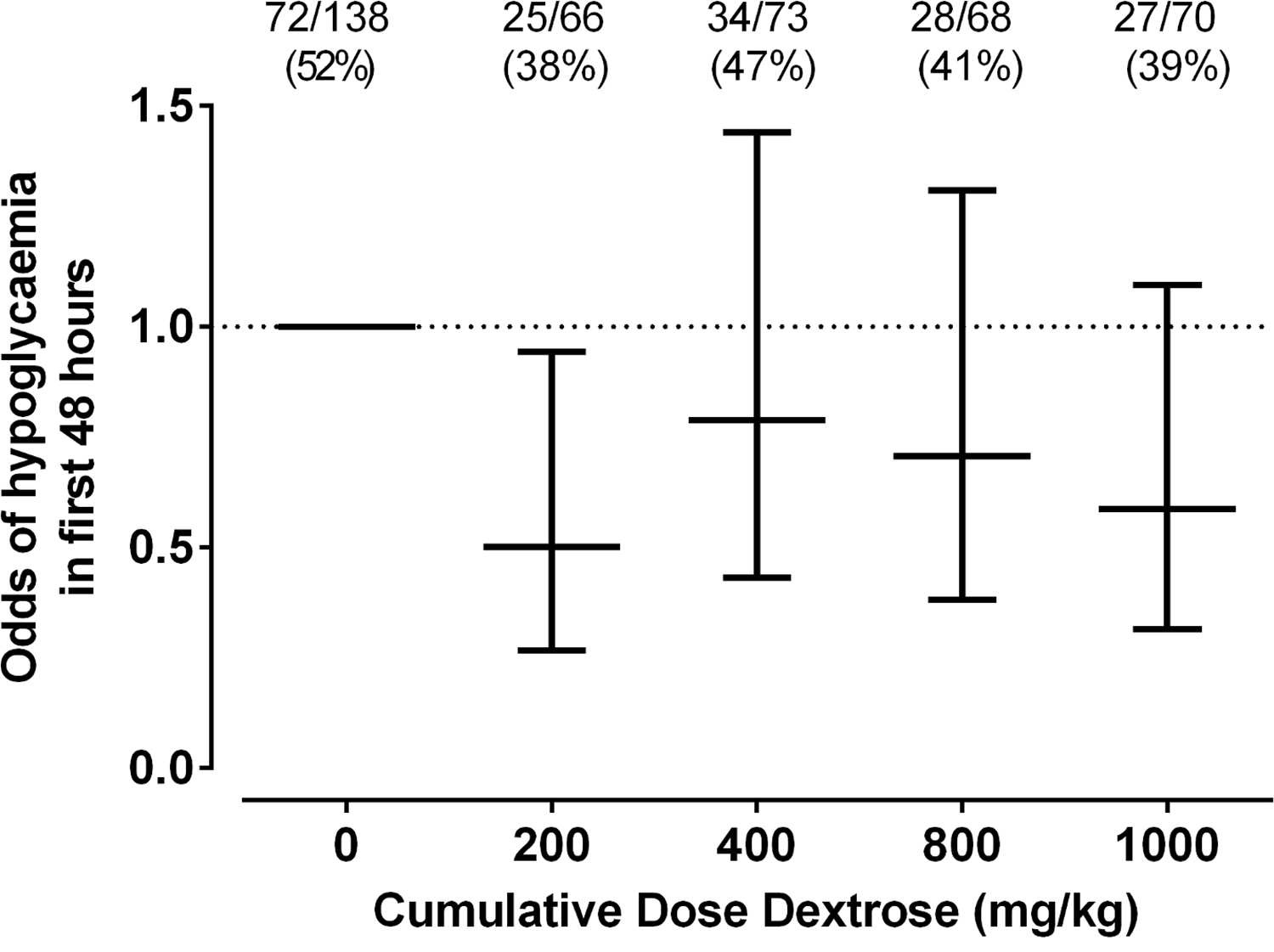
The only dose of dextrose that reduced the risk of hypoglycemia in the first 48 hours was 0.5 mL/kg which provides 200 mg/kg of dextrose which is the same as a bolus of IV dextrose when giving 2 mL/kg of D10W. Curiously using a higher dose or using multiple doses had no effect on reducing the risk. Based on a difference of 14% between placebo and this group you would need to treat roughly 7 patients with dextrose gel once to prevent one episode of hypoglycemia. Also worth noting is that admission to NICU was no different but if one restricted the reason for admission to hypoglycemia the difference was significant (13% vs 2% risk; p = 0.04). What was not seen here was a difference in rates of breastfeeding and much effect on use of formula.
Why Might These Results Have Occurred?
Insulin levels were not measured in this study but I truly wonder if the reason for hypoglycemia in the other groups may have been transient hyperinsulinemia from essentially receiving either a very large load of glucose (1 mL/kg groups) or effectively 4 boluses of glucose in the first 12 hours of feeding. Rebound hypoglycemia from IV boluses is a known phenomenon as insulin levels surge to deal with the large dextrose load and I can’t help but wonder if that is the reason that all but the single dose regimen had an effect. It is also worth commenting that with so many secondary outcomes in this study the p values needed to reach significance are likely much smaller than 0.05 so I would take the reduction in NICU admissions for hypoglycemia with a grain of salt although at least the trend is encouraging.
I wouldn’t change my practice yet and the authors do acknowledge in the article that a much larger study is now being done using the single dose of 0.5 mL/kg to look at outcomes and until that is published I don’t think a practice change is in order. What this study does reinforce though is that providing multiple doses of dextrose gel may yield diminishing returns. While the goal here was prophylaxis, I can’t help but think about the patients who are symptomatic and receive two or three gels and still wind up with an IV. Could it be the same rebound hypoglycemia at play?
We also have to acknowledge that even if this is an effective preventative strategy, is it in the best interests of the babies to all receive such treatment when at least in 6 babies they wouldn’t have needed any? Could such treatment simply be reserved as has been done for those who develop hypoglycemia? Those who question the safety of the ingredients such as dyes that are found in the product may want some long term safety data before this becomes routine in at risk babies but it won’t surprise me if such strategies become commonplace pending the results of the larger trial on the way.
by All Things Neonatal | Nov 3, 2016 | antenatal steroids, Neonatology, steroids, Uncategorized
In April of this year the ALPS trial results were published in the New England Journal of Medicine (Antenatal Betamethasone for Women at Risk for Late Preterm Delivery) and I took the time to review the paper at the time Antenatal Steroids After 34 weeks. Believe the hype? In the analysis I focused on an issue which was relevant at the time, being a shortage of betamethasone. In a situation when the drug of choice is in short supply I argued that while the benefits of giving steroid to women at risk of delivery between 34 0/7 to 36 6/7 weeks was there, if I had to choose (as I did at the time) I would save the doses for those at highest risk of adverse outcome. Since the blog post though a couple of things have come out in the literature that I believe are worth sharing as it could truly influence practice.
The American College of Obstetricians and Gynecologists, moved by the results of the ALPS trial issued the following recommendations (shortened in places).
- Betamethasone may be considered in women with a singleton pregnancy between 34 0/7 and 36 6/7 weeks gestation at imminent risk of preterm birth within 7 days.
- Monitoring of late preterms for hypoglycemia (already being done)
- Do not give in the setting of chorioamnionitis.
- Tocolysis or delayed delivery for maternal indications should not be done in order to to allow for administration of late preterm antenatal corticosteroids.
- Do not provide if the pregnancy was already exposed to antenatal corticosteroids.
The exclusions above such as twins and triplets, diabetic pregnancies and previous receipt of steroids were included since the study had not included these patients. As the ACOG states in the summary, they will be reviewing such indications in the future and providing recommendations. I would imagine that if I were in a US based practice then this post might seem like old news since many centres would have started doing this. Given that the readers of this blog are based in many different countries around the globe and at least in Canada this has not become commonplace I thought it would be worth the update!
I posted the abstract for this review on my Facebook page the other day and it certainly garnered a lot of interest. Some of my readers indicated the practice is already underway. I was curious what a systematic review would reveal about the topic since the ACOG was so moved by the ALPS study in particular. Perusing through the Society of Obstetricians and Gynecologists of Canada (SOGC) I can’t find any commentary on this topic and certainly there are no new clinical practice guidelines since the ALPS study landed on my desktop.
Here are the pooled results from 6 trials:
- Lower risk of RDS (relative risk 0.74, 95% confidence interval 0.61 to 0.91)
- Mild RDS (0.67, 0.46 to 0.96)
- Moderate RDS (0.39, 0.18 to 0.89)
- Severe RDS (0.55, 0.33 to 0.91)
- Transient tachypnea of the newborn (0.56, 0.37 to 0.86)
- Shorter stay on a neonatal intensive care unit (−7.64 days, −7.65 to −7.64)
So across the board patients who receive antenatal steroids after 34 weeks still continue to see a benefit but looked at a different way the real benefit of the intervention is easier to see and that is through looking at the number needed to treat (NNT). For those of you who are not familiar with this analysis, this looks at how many patients one would need to treat in order to avoid the outcome in 1 patient.
For the outcomes above as an example the NNT for RDS overall is about 59 while for TTN 31 patients. Severe RDS which is less common after 34 weeks you might expect to require more patients to treat to help 1 avoid the outcome and you would be correct. That number is 118 patients. It is interesting to look at the impact of steroids in pregnancies below 34 weeks (taken from the Cochrane review on the subject) as the NNT there is 23! If you were to break these benefits down from 23-27 weeks though where the risk of RDS is quite high the NNT would be even lower. Steroids help, no question to reduce neonatal complications but as you can see even when there is a reduction in risk for various outcomes, the number of women you need to treat to get one good outcome is quite different.
Some Discussion With Obstetrics Is Needed Here
As you read through this post you may find yourself saying “Who cares? if there is a benefit at all most moms would say give me the steroids!” The issue here has to do with long term outcome. To put it simply, we don’t know for this type of patient. We know clearly that for patients at high risk of adverse outcomes eg. 24 week infant, the reduction in risks of infection, NEC, PDA, BPD etc from receiving antenatal steroids translates into many long term benefits. What about the patient who say is 35 weeks and would have none of those risks? Yes we are avoiding some short term outcomes that let’s be honest can be scary for a new parent but what are we trading this benefit for. The concern comes from what we know about steroids impact on the developing brain. Steroids lead to a developmental arrest but in very preterm infants there is no doubt that the protective effect on all of these other outcomes more than offsets whatever impact there is there. Incidentally I wrote about this once before and the section of interest appears at the end of the relevant post Not just for preemies anymore? Antenatal steroids for elective c-sections at term. In the absence of these other conditions could there be a long term impact in babies 34 – 36 6/7? My suspicion is that the answer is no but discussion is needed here especially in the absence of an endorsement by our Canadian SOGC. Having said all that I expect the future will indeed see an expansion of the program but then I do hope that someone takes the time to follow such children up so we have the answer once and for all.

by All Things Neonatal | Sep 15, 2016 | Neonatal, Neonatology, Uncategorized
Campaign Closed October 13,2016! Thank you everyone for the $9359.00 raised!
Each day the number of people following these sites grows and at the time of this post, the largest following on Facebook has over 8200 people who receive the feed on a daily basis. That is nothing short of remarkable and I hope that each of you gets something out of my writings and postings. I recognise that each post may not “light it up” in your mind but if you get at least a few “a ha” moments along the way then I am very happy that you have found these sites!
What This Is Not!
As I begin hinting at money, many of you may be thinking “here we go”, he is finally asking for some payment for this site! To be clear I have no interest in personal financial gain from this hobby I have developed, but rather find my joy in sharing ideas, getting your feedback and helping to generate interest overall in topics pertaining to Neonatology. I have no intention of ever asking for such payment but that doesn’t mean that I might not want to help someone else. For those of you who make philanthropy a part of your lives you will know the joy that comes from helping others. Being able to help others need not take tremendous dollars per donor when you have many people banding together to help a cause. This is the power that I am hoping to harness through this initiative and make a difference in care to our babies in hospital.
For the past year and a half, I have put my fingers to the keyboard to hopefully share my knowledge and expertise with you about an industry I am so passionate about.
My Philanthropic Side
When I am not busy finding content for the sites or being a Neonatologist, I am quite dedicated to philanthropy. One thing people may not realise about our province/country is that the government helps out the best they can financially but with the heavy demands of our province, they can’t meet all the needs. That’s why I’m proud of my partnership with the Children’s Hospital Foundation of Manitoba. The Foundation’s donors have helped bridge the gap so our hospital doesn’t go without the specialised items they need. From ultrasounds, starting a breast milk depot, specialised pediatric equipment and funding a position to support Quality Improvement in our unit to a soon to be announced Family Support coordinator position and so much more. But now, I turn to you to help us make the next difference in our unit.
The other day as the Facebook page hit 8,000 followers a thought struck me. What if I asked everyone on the page to just give $1 towards the purchase of a piece of equipment for babies in our units?
Hold Their Hand
In the Neonatal Intensive Care Unit (NICU), they are watched closely to make sure they are getting the right balance of fluids and nutrition. Incubators or special warmers help babies maintain their body temperature. This reduces the energy the babies have to use to stay warm and allow them to use that energy elsewhere.
Premature babies need to receive good nutrition so they grow at a rate close to that of babies still inside the womb. Babies born under 38 weeks have different nutritional needs than babies born at full term (after 38 weeks). They often have problems feeding from a bottle or a breast. This is because they are not yet mature enough to coordinate sucking, breathing, and swallowing.
Many NICUs will give donor milk from a milk bank to high-risk babies who cannot get enough milk from their own mother. But because the baby must be kept at a certain temperature to stay warm, so does their milk. 
Thanks to the generous support of donors to the Children’s Hospital Foundation of Manitoba, 12 milk warmers have been purchased. However, we need 24 more warmers to keep up with demand. Each one costs $2,000 and will make a huge impact. An impact to help our babies get the nutrition they need at the temperature they require to survive and thrive.
So let’s hold their hand and let’s do it together! Has this journey of learning been worth at least $1 to you? If it has, then please help make a difference by giving at least $1. Giving more will only increase the power of this campaign! If you aren’t able to donate $1 or more, I ask that you share this post and challenge your friends to help make a difference to the over 1,000 patients we see a year. Click the link below to donate and make your difference today.
chfm.convio.net/help-hold-their-hand
by All Things Neonatal | Mar 9, 2016 | Neonatal, Neonatology, outcome, steroids, Uncategorized
It seems like a sensational title I know but it may not be as far fetched as you may think. The pendulum certainly has swung from the days of liberal post natal dexamethasone use in the 1990s to the near banishment of them from the clinical armamentarium after Keith Barrington published an article entitled The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs in BMC Pediatrics in 2011. This article heralded in the steroid free epoch of the first decade of the new millennium, as anyone caring for preterm infants became fearful of causing lifelong harm from steroid exposure.  Like any scare though, with time fear subsides and people begin asking questions such as; was it the type of steroid, the dose, the duration or the type of patient that put the child at risk of adverse development? Moreover, when death from respiratory failure is the competing outcome it became difficult to look a parent in the eye when their child was dying and say “no there is nothing more we can do” when steroids were still out there.
Like any scare though, with time fear subsides and people begin asking questions such as; was it the type of steroid, the dose, the duration or the type of patient that put the child at risk of adverse development? Moreover, when death from respiratory failure is the competing outcome it became difficult to look a parent in the eye when their child was dying and say “no there is nothing more we can do” when steroids were still out there.
Over the last decade or so, these questions in part have been studied in at least two important ways. The first was to ask whether we use a lower dose of dexamethasone for a shorter period to improve pulmonary outcomes without adverse neurodevelopment? The target population here were babies on their way to developing chronic lung disease as they were ventilated at a week of age. The main study to answer this question was the DART study. This study used a very low total dose of 8.9 mg/kg of dexamethasone given over ten days. While the study was stopped due to poor recruitment (it was surely difficult to recruit after the 2001 moratorium on steroids) they did show a benefit towards early extubation. This was followed up at 2 years with no difference in neurodevelopmental outcomes. Having said that the study was underpowered to detect any difference so while reassuring it did not prove lack of harm. Given the lack of evidence showing absolute safety practitioners have continued to use post natal steroids judiciously.
The second strategy was to determine whether one could take a prophylactic approach by providing hydrocortisone to preterm infants starting within the first 24 hours to prevent the development of CLD. The best study to examine this was by Kristi Watterberg in 2004 Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Strangely enough the same issue of early stoppage affected this study as an increased rate of spontaneous gastrointestinal perforation was noted leading to early closure. The most likely explanation is thought to be the combination of hydrocortisone and indomethacin prophylaxis which some centres were using at the same time. An interesting finding though was that in a subgroup analysis, infants with chorioamnionitis who received hydrocortisone had less incidence of chronic lung disease. (more on this later) Although this of course is subject to the possible bias of digging too deep with secondary analyses there is biologic plausibility here as hydrocortisone could indeed reduce the inflammatory cascade that would no doubt be present with such infants exposed to chorioamnionitis in utero.
Has the answer finally come?
The DART study at 360 patients was the largest study to date to look at prophylaxis as a strategy. That is until this past week. The results of the PREMILOC study have been published which is the long awaited trial examining a total dose of 8.5 mg/kg of hydrocortisone over 10 days. We can finally see the results of a trial without the complicating prophylactic indomethacin trials interfering with results. Surprisingly this study was also stopped early (a curse of such trials?!) due to financial reasons this time. Prior to stoppage though they managed to recruit 255 to hydrocortisone and 266 to control groups. All infants in this study were started on hydrocortisone within 24 hours of age and the primary outcome in this case was survival without BPD at 36 weeks of age.
All infants were less than 28 weeks at birth and therefore had a high risk of the combined outcome and despite the study being stopped early there was indeed a better outcome rate in the hydrocortisone group (60% vs 51%). Another way of looking at this is that to gain one more patient who survived without BPD you needed to treat 12 which is not bad at all. What is additionally interesting are some of the findings in the secondary analyses.
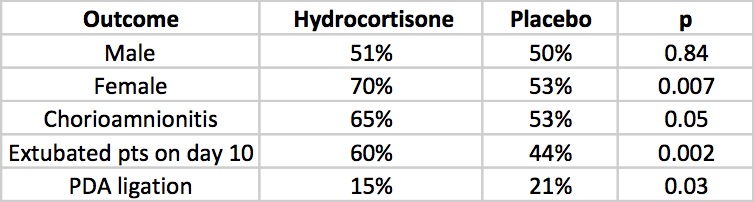
The lack of a difference in males may well reflect the biologic disadvantage that us males face overcoming any benefit from the hydrocortisone. In fact for the females studied the number needed to treat improves to 6 patients only! Short term outcomes of less ventilation are sure to please everyone especially parents. Lastly, a reduction in PDA ligation is most probably related to an antiprostaglandin effect of steroids and should be cause for joy all around. Lastly, a tip of the hat to Dr. Watterberg is in order as those infants who were exposed to chorioamnionitis once again show that this is where the real benefit may be.
But what about side effects?
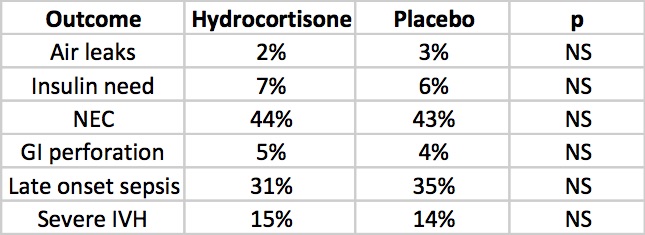
The rate of NEC is quite high but is so for both groups but otherwise there is nothing much here to worry the reader. Once and for all we also see that by excluding concurrent treatment with indomethacin or ibuprofen the rate of GI perforation is no different this time around. Reassuring results indeed, but alas the big side effect, the one that would tip the scale towards us using or abandoning treatment has yet to be presented. Steroids no doubt can do great things but given the scare from 2001 we will need to see how this cohort of babies fares in the long run.
The follow-up is planned for these infants and the authors have done an incredible job of recruiting enough patients to make the results likely believable. I for one can’t wait to see what the future holds. If I was a betting man though I would say this ultra low dose of hydrocortisone may be just the thing to bring this therapy finally into the toolbox of neonatal units worldwide. We have been looking for the next big thing to help improve outcomes and good old hydrocortisone may be just what the doctor ordered.
by All Things Neonatal | Mar 3, 2016 | antenatal steroids, Neonatal, Neonatology, Uncategorized
What a hard topic to resist commenting on. This was all over twitter and the general media this week after the New England Journal published the following paper; Antenatal Betamethasone for Women at Risk for Late Preterm Delivery. The fact that it is the NEJM publishing such a paper in and of itself suggests this is a top notch study…or does it?
In case the idea of giving antenatal steroids after 34 weeks sounds familiar it may be so as I wrote about the use of such an approach prior to elective c-section in a previous post; Not just for preemies anymore? Antenatal steroids for elective c-sections at term.
Is there a benefit to giving antenatal steroids from 34 0/7 – 36 5/7 weeks?
That is the central question the authors here sought to answer. Would women who had a high risk of delivering during this time period have less risk of a composite primary outcome of treatment in the first 72 hours (the use of continuous positive airway pressure or high-flow nasal cannula for at least 2 hours, supplemental oxygen with a fraction of inspired oxygen of at least 0.30 for at least 4 hours, extracorporeal membrane oxygenation, or mechanical ventilation) or stillbirth or neonatal death within 72 hours after delivery.
On the surface this seems like a very worthwhile set of outcomes to look at and the authors found in the end some pretty remarkable findings in a total of 2827 women randomized to placebo or betamethasone.
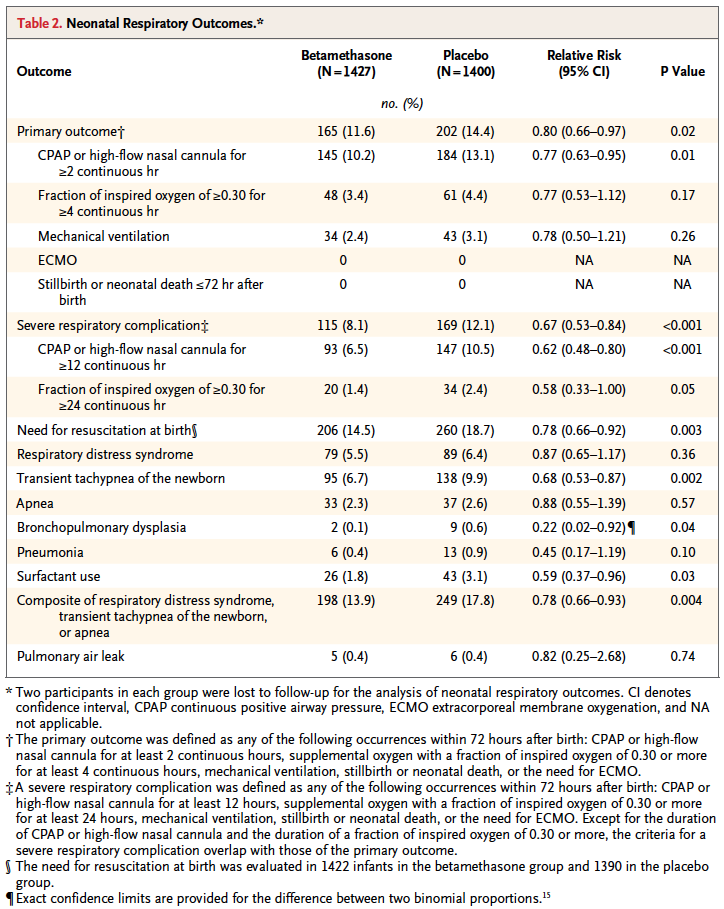
Looking at the results one sees that the primary outcome showed a significant difference with 2.8% less infants experiencing these conditions. However, when one looks at the details the only contributor to this difference was the need for CPAP or HFNC for >= 2 hours. A need for over 30% FiO2 for > 4 hours was also not different. No differences were noted in mechanical ventilation, ECMO, deaths whether stillbirths or neonatal deaths. Curiously, significant differences for secondary outcomes were seen with incidence of severe respiratory distress, and need for CPAP for over 12 hours.
These results are not truly that surprising at least for the primary outcome as if you asked most people working in the field of Neonatology how likely death, need for ECMO or even mechanical ventilation are from 34 – 36 weeks they would tell you not very likely. The other thing to consider is that the only real significant difference was noted for infants needing CPAP or HFNC for at least 2 hours. While this would interrupt maternal infant bonding, it wouldn’t necessarily mean an admission but rather in some cases observation and then transfer to the mother’s room.
Is it worth it?
To answer this question you need to know the best and worst case scenarios I suppose. Based on the reduction of 2.8%, you would need to treat 35 women with betamethasone to avoid the primary outcome but of course there is a range based on the confidence intervals around this estimate. The true estimate lies somewhere between 18 – 259 to avoid the outcome. Having said that, the estimate to avoid severe distress is 25 patients with a range of 16 – 56 which is pretty good value. In a perfect world I would probably suggest to women that there seems to be a benefit especially if one notes that in this study only 60% of the women received 2 dose of betamethasone so if rates of administration were higher one might expect and even better outcome. Ah but the world is not perfect….
There is only so much betamethasone to go around.
I find it ironic but the same day that this article came across my newsfeed so did a warning that we were about to run out of betamethasone vials in a certain concentration and would need to resort to another manufacturer but that supply may also run out soon as well. The instructions were to conserve this supply in the hospital for pregnant women.
In Canada as reported by the Canadian Neonatal Network in 2010, 38.1% of babies admitted to NICUs were below 34 weeks. Given that all babies would be admitted to NICUs at this gestational age and below that likely represents the percentage of births in those ages. An additional 31.8% or almost an equal number of babies will be born between 34 0/7 to 37 0/7 weeks meaning that if we were to start treating women who were deemed to be at risk of preterm delivery in that age range we would have a lot of potential women to choose from as these are the exact women in this strata who actually delivered early in Canada.
If I am forced to choose whether to give betamethasone to the mothers under 34 weeks or above when the resource we need is in scarce supply I don’t think there is much choice at all. Yes, this article comes from a reputable journal and yes there are some differences some of which are highly significant to consider but at least at this time my suggestion is to save the supply we have the babies who will benefit the most.
by All Things Neonatal | Jan 7, 2016 | Innovation, Neonatology, Prematurity
In the spirit of full disclosure I have to admit I have never placed a laryngeal mask airway (LMA) in a newborn of any gestational age. I have played with them in simulated environments and on many occasion mentioned that they are a great alternative to an ETT especially in those situations where intubation may not be possible due to the skill of the provider or the difficulty of the airway in the setting of micrognathia for example.
In recent years though we have heard of examples of surfactant delivery via these same devices although typically these were only case reports. More recently a small randomized study of 26 infants by Attridge et al demonstrated in the group randomized to surfactant administration through an LMA that oxygen requirements were reduced after dosing. This small pilot provides sufficient evidence to show that it is possible to provide surfactant and that at least some gets into the airway of the newborn. This proof of concept though while interesting, did not answer the question of whether such delivery of surfactant would be the same or better than through an ETT. As readers of my blog posts know, my usual stance on things is that the less invasive the better and as I look through the literature, I am drawn to concepts such as this to see if they can be added to our toolbox of non or less invasive strategies in the newborn.
A Minimally Invasive Technique For The Masses?
This past month, a small study by Pinheiro et al sought to answer this question by using 61 newborns between 29 0/7 – 36 6/7 weeks and greater than 1000g and randomizing them to either surfactant via the INSURE technique or LMA. I cannot stress enough so will get it out of the way at the start that this strategy is not for those <1000g as the LMA is not designed to fit them properly and the results (to be shown) should not be generalized to this population. Furthermore then study included only those infants who needed surfactant between 4 – 48 hours of age, were on CPAP of at least 5 cm H2O and were receiving FiO2 between 30 – 60%. All infants given surfactant via the insure technique were premedicated with atropine and morphine while those having an LMA received atropine only. The primary outcome of the study was need for subsequent intubation or naloxone within 1 hour of surfactant administration. The study was stopped early after an interim analysis (done as the fellow involved was finishing their fellowship – on a side note I find this an odd reason to stop) demonstrated better outcomes in the group randomized to the LMA.
Before we get into the results let’s address the possible shortcomings of the study as they might already be bouncing around your heads. This study could not be blinded and therefore there could be a significant bias to the results. The authors did have predetermined criteria for reintubation and although not presented, indicate that those participating stuck to these criteria so we may have to acknowledge they did the best they could here. Secondly the study did not reach their numbers for enrolment based on their power calculation. This may be ok though as they found a difference which is significant. If they had found no difference I don’t think I would be even writing this entry! Lastly this study used a dose of surfactant at 3 mL/kg. How well would this work with the formulation that we use BLES that requires 5 mL/kg?
What were the results?
| Intervention Failure |
LMA Group |
ETT group |
p |
| Any failure |
9 (30%) |
23(77%) |
<0.001 |
| Early failure |
1 (3%) |
20 (67%) |
<0.001 |
| Late failure |
8 (27%) |
3 (10%) |
0.181 |
What do these results tell us? The majority of failures occurred within an hour of delivery of surfactant in the ETT group? How does this make any sense? Gastric aspirates for those in the LMA group but not the INSURE group suggest some surfactant missed the lung in the former so one would think the intubation group should have received more surfactant overall however it would appear to be the premedication. The rate of needing surfactant afterwards is no different and in fact there is a trend to needing reintubation more often in the LMA group but the study was likely underpowered to detect this difference. Only two patients were given naloxone to reverse the respiratory depressive effects of morphine in those given the INSURE technique so I can’t help but speculate that if this practice was more frequent many of the reintubations might have been avoided. This group was quite aggressive in sticking to the concept of INSURE as they aimed to extubate following surfactant after 5 – 15 minutes. I am a strong advocate of providing RSI to those being electively intubated but if the goal is to extubate quickly then I believe one must be ready to administer naloxone soon after extubation if signs of respiratory depression are present and this did not happen effectively in this study. Some may argue those getting the INSURE technique should not be given any premedication at all but that is a debate that will go on for years I am sure but they may have a valid point given this data.
Importantly complications following either procedure were minimal and no different in either group.
Where do we go from here?
Despite some of the points above I think this study could prove to be important for several reasons. I think it demonstrates that in larger preterm infants it is possible to avoid any mechanical ventilation and still administer surfactant. Many studies using the minimally invasive surfactant treatment (MIST) approach have been done but these still require the skill of laryngoscopy which takes a fair bit of skill to master. The LMA on the other hand is quite easy to place and is a skill that can be taught widely. Secondly, we know that even a brief period of over distension from PPV can be harmful to the lung therefore a strategy which avoids intubation and direct pressure to the lung may offer some longer term benefit although again this was not the study to demonstrate that.
Lastly, I see this as a strategy to look at in more rural locations where access to highly skilled level III care may not be readily available. We routinely field calls from rural sites with preterm infants born with RDS and the health care provider either is unable to intubate or is reluctant to try in favour of using high flow oxygen via mask. Many do not have CPAP either to support such infants so by the time our Neonatal Transport team arrives the RDS is quite significant. Why not try surfactant through the LMA? If it is poorly seated over the airway and the dose goes into the stomach I don’t see them being in any worse shape than if they waited for the team to arrive. If some or all of the dose gets in though there could be real benefit.
Might this be right for your centre? As we think about outreach education and NRP I think this may well become a strong reason to spend a little more time on LMA training. We may be on to something!







 Like any scare though, with time fear subsides and people begin asking questions such as; was it the type of steroid, the dose, the duration or the type of patient that put the child at risk of adverse development? Moreover, when death from respiratory failure is the competing outcome it became difficult to look a parent in the eye when their child was dying and say “no there is nothing more we can do” when steroids were still out there.
Like any scare though, with time fear subsides and people begin asking questions such as; was it the type of steroid, the dose, the duration or the type of patient that put the child at risk of adverse development? Moreover, when death from respiratory failure is the competing outcome it became difficult to look a parent in the eye when their child was dying and say “no there is nothing more we can do” when steroids were still out there.



