by All Things Neonatal | Aug 17, 2015 | Neonatology, neurology
I suppose I am as guilty as anyone with respect to the evolution of this story. Twenty years ago early and late cranial ultrasounds were employed to give clinicians some guidance as to what to tell parents about their growing preterm infant. Blood seen on early ultrasounds might influence aggressiveness of care or help us remain vigilant for the development of evolving hydrocephalus. Similarly, late cranial ultrasounds might detect periventricular leukomalacia or other white matter injury and alert us to a higher risk of cerebral palsy. Then things changed with the widespread availability of MRI. As I have mentioned to countless parents, MRI compared to ultrasound is like comparing an old 1 megapixel camera to a modern day 18 megapixel. The resolution is simply much better and with it our ability to see things that previously escaped our detection.
As utilization of MRI increased so did publications pertaining to its findings. With the enhanced clarity, white matter lesions that were previously missed became evident and imaging of the posterior fossa or specifically the cerebellum improved as described by Tam et al. Bleeds in this area that were previously missed could now be readily seen, although more recently use of the mastoid view when performing cranial ultrasound may pick up many significant lesions as well. Fear in the medical community arose in 2007 after a paper entitled Does Cerebellar Injury in Premature Infants Contribute to the High Prevalence of Long-term Cognitive, Learning and Behavioral Disability in Survivors by Limperopoulos et al. In this study of 86 patients overall, presence of cerebellar injury with or without supretentorial lesions was associated with neurodevelopmental impairment in 66% versus 5% of age matched controls. Given that cranial ultrasound may miss such cerebellar lesions it seemed prudent to begin imaging all high risk patients at term to determine if there was a significant risk of long-term disability. As Neonatologists we are often asked if a families baby will be ok and so with the ability to obtain such information why would one not pursue such imaging?
With this finding, utlilization of MRI at term equivalent age became widespread including our own centre. In hindsight however this practice was not thought out carefully and the ramifications of this decision were significant. One of the most common things that we teach to medical students is the practice to ask themselves the following question before they do any diagnostic test.
“How will the results of this test influence your management?”
If the result is positive what will you do and similarly if it is negative will this help you in any way. It is the answers to these questions that leave me with a desire to travel back in time and influence my colleagues to choose a different path than we did. The problem lies in the meaning of the test, either positive or negative and what if anything we will do with the result that is different than if we had not known in the first place. This issue was recently highlighted in a large trial from the NICHD entitled Neuroimaging and Neurodevelopmental Outcome in Preterm Infants. The results of the MRI studies compared with cranial ultrasound demonstrated again the superior capabilities of MRI to diagnose cerebellar bleeds but as can be seen in the following table, the absence of any lesions on MRI does not mean the parents should be told all will be well with their infant.
|
Severity of WMA |
|
Normal |
Mild |
Moderate |
Severe |
| Cognitive score < 70 |
4.1 |
4.3 |
10.5 |
22.2 |
| Corgnitive score < 85 |
20.4 |
18.2 |
29.9 |
61.1 |
| Any CP |
2 |
5.4 |
5.9 |
61.1 |
| Moderate to severe CP |
0 |
1.9 |
1.5 |
55.6 |
| NDI |
4.1 |
6.2 |
10.5 |
55.6 |
From the table you will note though that with increasing injury, the risk of adverse outcomes increase as well. On the surface this would imply that the information may be important, as we may be able to tell the parents that we are concerned if the lesions are more significant. What would we do with this information though that is not already being done? Herein lies the major issue in all of this.
Modern NICU care entails having a follow-up program for all patients designated as high risk. In the case of our centre this means having a multidisciplinary team evaluating all infants born under 1500 g. Infants who in follow-up demonstrate deficits whether they be cognitive, motor or both are provided with the support they need to address these deficits. Whether the family knows that there is an abnormality in the MRI or not does not influence the trajectory they are on in terms of evaluation and suggestions for any therapies that are needed. The use of the MRI fails the test that I mentioned earlier. If it is normal the child may still have deficits or if abnormal the infant could still be unimpaired. The fact that the degree of severity on the MRI being more predictive of an abnormal outcome does nothing more than provide families with something to worry about pending the formal testing when they are seen in follow-up.
You may think I am being paternalistic to a certain degree but these beliefs were illustrated by a family after their experience at St. Justine in Montreal. I suspect that the practice has changed there but at the time as was done in Winnipeg, MRIs were done at term equivalent age for all infants under a certain weight. The case of Maren, a surviving twin, born at 25 weeks gestational age was one in which she was diagnosed with a cerebellar hemorrhage on MRI. The family took to the internet as many parents do and uncovered the paper on cerebellar bleeds referenced above and spent the next several months in anguish as they waiting for the results of the follow-up testing and moreover seeing how she would develop. I think this quote from the article sums it up very well as to what the test meant for them.
“In our case, Maren’s MRI gave us no information about what she is like today, it served only to completely terrify us. Maren is now two and a half with no disabilities”
This past month the AAP released a “Choosing Wisely” recommendation on this practice and suggests as I have that it be stopped. I hope for the sake of families everywhere this is widely adopted. Sometimes it is just better to leave things well enough alone when all that your test will do is create more anxiety for parents who have already experienced enough.

by All Things Neonatal | Jul 30, 2015 | Neonatal, Neonatology, Pregnancy
The benefits of antenatal steroids before preterm birth have been clearly demonstrated in the literature and have been nicely summarized in a Cochrane Review. From this report the evidence is clear. Treatment with antenatal corticosteroids prior to preterm birth is associated with an overall reduction in neonatal death (relative risk (RR) 0.69, 95% confidence interval (CI) 0.58 to 0.81, 18 studies, 3956 infants), RDS (RR 0.66, 95% CI 0.59 to 0.73, 21 studies, 4038 infants), cerebroventricular haemorrhage (RR 0.54, 95% CI 0.43 to 0.69, 13 studies, 2872 infants), necrotising enterocolitis (RR 0.46, 95% CI 0.29 to 0.74, eight studies, 1675 infants), respiratory support, intensive care admissions (RR 0.80, 95% CI 0.65 to 0.99, two studies, 277 infants) and systemic infections in the first 48 hours of life (RR 0.56, 95% CI 0.38 to 0.85, five studies, 1319 infants).
While it is clear that corticosteroid administration prior to 37 weeks has great benefit, the question is whether these benefits might actually extend to 37 and 38 weeks. It has been known for some time that having an elective c-section before 39 weeks exposes the infant to an increased risk of pulmonary morbidity and NICU admission. In 2009 Tita At et al studied 24077 repeat elective c-sections at term finding that 36% were performed prior to 39 weeks. The findings conclusively demonstrated that delivery at 37 and 38 weeks increased the likelihood of a composite outcome of death or respiratory complications, treated hypoglycemia, newborn sepsis and admission to the NICU. 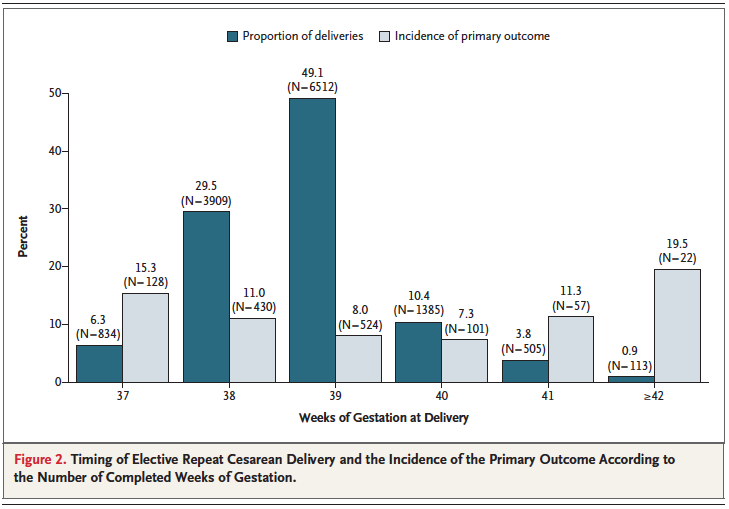 Interestingly one can also see that after 40 weeks these complications rose again. Post term deliveries are not without their consequences either.
Interestingly one can also see that after 40 weeks these complications rose again. Post term deliveries are not without their consequences either.
Broken down by outcome, it is also clear that each component has an increased risk at both 37 and 38 weeks compared to delivery at 39 or 40 weeks.
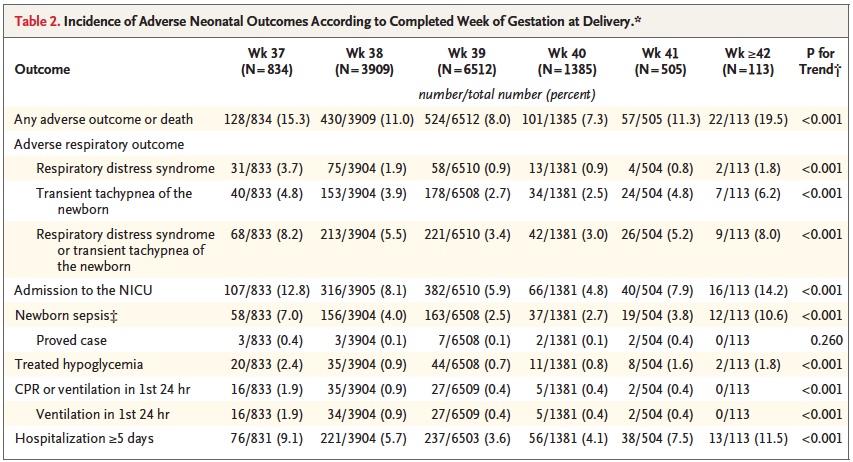
With such increased risk this practice has been discouraged by many obstetrical organizations including the American College of Obstetricians and Gynecologists.
Knowing that there is clear benefit to providing corticosteroids before 37 weeks, it was only a matter of time before someone would test the hypothesis that treatment of women having an elective c-section in would reduce the incidence of respiratory complications such as TTN and RDS. Surprisingly there is really only one relevant study on this subject performed by P. Stutchfield et al in 2005 entitled Antenatal Betamethasone and Incidence of Neonatal Respiratory Distress After Elective Caesarean Section: A Pragmatic Trial. The trial provided betamethasone as a single course of two doses 24 hours apart starting 48 hours before a planned c-section with 998 participants in total. 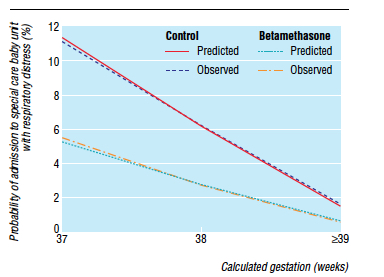
The primary outcome in this trial was admission to NICU with respiratory distress. While the study was unblinded, the results were impressive and shown in the figure to the right indicating that below 39 weeks there was a significant difference in likelihood of admission for respiratory distress if women were treated with betamethasone prior to elective delivery via c-section. In terms of effectiveness this translates to the need to treat 37 women at 37-38 weeks with betamethasone to prevent one admission for respiratory distress to NICU. Eighty percent of the newborns in the control group had TTN versus RDS so I would expect you would need to treat about 200 women to prevent one case of RDS at this gestational age. Is it worth it? I suspect if you told parents that you could prevent hospital admission of their newborn at all many would choose to do so. There is another side to this though that one must consider and that side is the impact on neurodevelopment.
Corticosteroids work by overcoming the maternal capacity to break down cortisol by a placental enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HDS-2). Furthermore the corticosteroids used (betamethasone and dexamethasone) are resistant to degradation by this enzyme. In the brain this enzyme exists as well and has increased activity such that levels of active cortisol in the brain are at a minimum. In animal models, high levels of glucocorticoids cause decreased brain differentiation with reduced neurogenesis. These processes are likely to be similar in humans given the presence of the same enzyme which has little effect in inactivating these synthetic medications.
Even with this knowledge, we as health care providers freely recommend antenatal steroids to women at risk of preterm birth for all the benefits outlined at the start of this post. Preterm infants are at significant risk of IVH, PVL, NEC, PDA and many other conditions which in and of themselves have been linked with adverse neurodevelopment. It is the avoidance of these outcomes which likely explains why corticosteroid administration with it’s known effect on the developing brain leads to improved neurodevelopmental outcome. The challenge here is that can we extrapolate this to the 38 and 39 week fetus? I would suggest that this is not the case as the risks of the conditions leading to neurodevelopmental impairment are magnitudes less. We are then exposing these fetuses to the potential harm or glucocorticoids without the benefit of reducing the conditions that matter to outcome. On the other side of the scale is a reduction in TTN/RDS and admission to the NICU but is it worth treating 37 mothers to avoid this with the heavy weight on the other side?
If you believe I am making some unfair assumptions it is worth seeing what happened to the patients in the 2005 study by Stuchfield when they were followed up between 8 – 15 years of age. The study used a questionnaire to address a number of outcomes related to education, atopy and behaviour. The response rate for the study was only 51% of the original cohort so any conclusions must be taken with a grain of salt. That being said the authors state that there were no differences in outcome or difference in rates of asthma and atopy. In their conclusion they affirm that based on the lack of differences in long-term outcomes but with improved short-term respiratory status at birth steroids should be provided before elective c-sections. Curiously though the authors do not address an interesting finding shown in table 2 from the article.
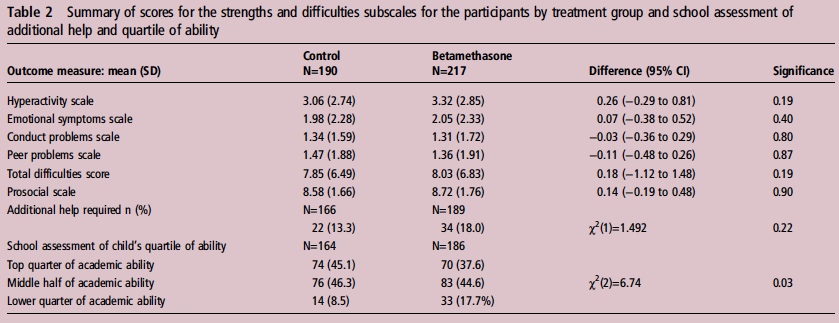
Looking at the bottom section pertaining to the school’s assessment of a child’s academic ability, less children in the steroid group performed at the top quarter of the class and twice as many children were in the lower quarter of the class. To me at least it seems disingenuous to claim no differences were seen when clearly here is a difference based on a third-party (the teacher) that is significant. The academic purists will be quick to point out that this is a secondary analysis and not the primary outcome specifically of the study and that the numbers are small. Additionally one can also argue that at a 51% response rate we are missing a great deal of outcomes. Furthermore it may well be that when it comes to surveys, those who have concerns about their participation in the study may be more apt to complete it skewing the results.
I will allow all these arguments as it really helps to support my conclusion on all of this. There is very little data out there on the benefit of providing antenatal steroids at term before elective c-section. The data out there for long-term effects does show a concern regarding school performance and the exposure in this case is to medication which is known to have effects on the developing brain. That data though is suspect as well given the issues raised in the above paragraphGiven the number of women that need to be treated to avoid one admission for respiratory distress and with the above mentioned concerns I believe more studies are needed to determine whether this is worth instituting as standard practice. Finally, any future studies will need to address in a prospective manner using a large number of patients whether there is indeed any impact on development in the long-term from such practice.
by All Things Neonatal | Jul 27, 2015 | Neonatal, Neonatology
Go to any NICU these days and you will no doubt hear about the toxicity of oxygen. Oxygen as we say is a drug and like any such product has both good and bad effects. On the good side is the ability to increase the fraction of inspired oxygen to deal with transient changes in observed saturations. In extreme cases where the patient is desaturating into the 20’s and 30’s with accompanying bradycardia this can be life saving. On the other hand the “bad” is related to toxicity from oxygen free radicals which can increase rates of ROP, BPD and in the case of resuscitation with O2 vs room air may increase the chances of death.
It is for this reason that NICU teams pay particular attention to saturation targeting. The optimal goal remains elusive as oxygen restriction to 85-89% has been linked to higher mortality as in the Support Trial (full article) while higher saturations may increase the rate of ROP and BPD. Many units are falling somewhere in the middle such as goals of 90-95% or as in our units 88-93%. If during your visit to an NICU you were to observe the nurses at the bedside you would see them or the Respiratory Therapists tweaking the oxygen up and down many times a day as they strive to keep the saturations within these ranges. As a Neonatologist I greatly appreciate the efforts of everyone on the team who try to keep maintain these goals but in the end how do they really do?
This was the subject of a 2006 paper by Hagadorn et al. Eighty four infants from 14 centres all less than 28 weeks were enrolled in this study looking at how successful staff were at keeping infants within a desired range. The findings were somewhat discouraging in that 16-64% of the time saturations were in range, while 20-73% of the time they were above range depending on the centre studied. In a different study by Laptook et al published in the same year, the results were not much better with saturations higher than goal about 15% of the time and lower by nearly 26% of the time. What impact might swings in oxygen saturation have on the brain when the saturations are low and similarly on rates of BPD and ROP when the tendency is to overshoot the goals? There is no doubt that everyone is trying to do a good job but how deflating is it to nursing and other staff members when despite their best efforts they are only in range about 60% of the time?
Fortunately I believe change is coming. With improvements in technology it is now possible to have a closed loop system in which a patient who is receiving oxygen has their saturation measured and the information via a feedback loop triggers an automated response. This response either raises or lowers the FiO2 in an automatic way which eliminates the need for health care staff to make such changes. Such technology is actually not that new as it was tested in 2001 by Claure et al and was found to be at least as effective as manual changes by a dedicated nurse. Several other small studies followed, all demonstrating an improved accuracy in maintaining target saturations. This July the same group published the following article Automated versus Manual Oxygen Control with Different Saturation Targets and Modes of Respiratory Support in Preterm Infants. This study compared the accuracy in maintaining saturations using a target of 91-95% and another of 89-93% with 80 patients participating. Patients in this study received both invasive and non-invasive ventilation. When comparing the two target ranges the automatic adjustments showed greater benefit with the results being 62 +/- 17% vs 54 +/- 16% for the 91-95% range and 62 +/- 17% vs 58 +/- 15% in the lower range. Both of these results were statistically different with p values of <0.001. When looking at episodes of significant hypoxemia as defined by a saturation < 80% the findings were interesting showing in the high range 13 vs 4 and in the low range 15 vs 4 favouring the use of autosaturations. The authors were surprised at the lack of large disparity in the accuracy between manual and auto adjustment of FiO2 but this may be related to the Hawthorne effect. Since the nurses in this study were not blinded to the intervention they may have changed their behaviour in essence to try and prove that they were just as good. How this would translate into a real world situation when a study is not being performed I suspect would favour the automated system more.
Perhaps the most interesting part of the study though was the number of Manual FiO2 adjustments per 24 hours between the two groups. For the 89%-93% SpO2 target range this was 1 [0-3] vs 102 [73-173], P < .001 and for the 91%-95% SpO2 target range 1 [0-3] vs 109 [79-156], P < .001. For me this is the crux of the discussion. In a twenty four hour period there is a reduction of about 100 interventions using the saturation range similar to our own. Take note as well that some patients required over 150 interventions in 24 hours as 100 was the mean! While the targeting is improved somewhat with the use of the auto FiO2 adjustment this is where the biggest benefit to the patient lies as I see it. Nurse and respiratory therapists are very busy on any given shift. One hundred adjustments a day translates into 4 changes in FiO2 per hour on average. Although not measured I can’t help but wonder what impact these interruptions have on the rest of the care for a given patient. What is the “ripple effect” of such interruptions in a person’s train of thought. Could this translate into a med error or delay care for another patient to whom the nurse is responsible for as well? If one didn’t have to pay as much attention to the FiO2, what other goals could their attention be put towards? Might this provide them with more time to educate parents, do skin care, or a host of other responsibilities which in the current state get interrupted every time an alarm goes off.
As this technology is improved I see this being a welcome addition to the NICU. As with anything though that promises a hands free environment it will be essential to have an override built in. Furthermore we can never take our eyes off the patient as no matter how safe these systems may be there is always the chance that a computer will freeze as we all know. The impact of such a “glitch” in the algorithm that these systems use could be catastrophic to outcome so as good as these may be we will always need the human presence to ensure that what we think we are getting from the system is actually there.

by All Things Neonatal | Jul 25, 2015 | Neonatology, resuscitation
Anyone who attends the delivery of high risk newborns will eventually encounter a baby who is born “flat”. Most of these babies will respond to stimulation and for those that don’t, the vast majority of the remaining group will come around with the use of positive pressure ventilation. The remaining infants thankfully are the rare group but these are the ones that have the highest likelihood of dying or being severely impaired and therefore leave little room for error in their resuscitation if we hope to achieve a good outcome.
In 1816 Rene Theophile Hyacinthe Laennec  invented the stethoscope to listen to the sounds of the heart and lungs. It is hard to believe that this coming year marks 200 years since that discovery and even harder to believe that when it counts most, this device still remains the best tool at your fingertips. When resuscitation has moved past the initial steps there is really nothing that can replace it’s accuracy when the going gets tough.
invented the stethoscope to listen to the sounds of the heart and lungs. It is hard to believe that this coming year marks 200 years since that discovery and even harder to believe that when it counts most, this device still remains the best tool at your fingertips. When resuscitation has moved past the initial steps there is really nothing that can replace it’s accuracy when the going gets tough.
The most recent NRP guidelines recommend that all neonates who are receiving PPV have a preductal O2 pulse oximeter placed and oxygen titrated to the amount required to keep saturations within a range based on the age of the patient. Heart rate will be displayed when such monitoring is applied as well as when chest leads are placed giving you two readings of heart rate to compare. Such comparisons often prove useful when trying to determine if the displayed saturation is an adequate result. When looking at the recommendations from the NRP there is the clear statement that the best way of determining the heart rate is through auscultating the precordial pulse but does this always happen?
The answer as you might expect is no. Quite often during resuscitation I am told what the heart rate is based on the monitor. There is a danger in relying on such technology as you will see below. Recently I was called to the resuscitation area after the delivery of a non-vigorous infant. The patient was not responding to positive pressure ventilation as determined by the colour of the patient , which was dark purple despite confirmation of tube placement by end tidal capnography. The team was guiding their continued PPV without compressions based on the monitor which was showing a heart rate of 120-130 beats per minute and the belief that they had palpated a pulse. After hearing this and recognizing that the patient before me did not fit the reading on the monitor I reached for the stethoscope. The finding of no audible heart rate prompted us to start chest compressions.
What concerned me about this case was that the presence of technology actually hindered the institution of advanced resuscitation techniques. In the last few years much of the attention in the NRP program has shifted to monitoring during resuscitation. There have been many investigating the role of O2 saturation targeting, comparisons of chest leads versus pulse oximeters for acquisition of heart rates and stressing of the importance of attaching the probe to the patient and then to the monitor to improve signal acquisition times. This patient was in Pulseless Electrical Activity (PEA) which went unrecognized due to an adequate heart rate being visualized on a monitor in the context of a non-congruent clinical exam. PEA is a state in which the heart is still experiencing electrical conduction but there is not enough contractility to eject blood.
Another interesting aspect to this case was the claim that the infant had an adequate pulse. When I say claim I don’t mean that I believe the person in this case was lying but rather they believed they felt a pulse. As with many other posts I felt obliged to ask the question “How accurate is assessment of a pulse in a resuscitation?” As much as we would like to think we all stay calm under pressure there is no doubt that when it counts most and our heart is racing from our sympathetic nervous system on overdrive, we may experience the opposite state. A nicely done study addressed such accuracy in 2009 using patients who were on heart lung bypass. 209 doctors and nurses were asked to blindly assess presence or absence of pulse with the manipulation of pulsatility by using the presence of a left ventricular assist device or not. The findings of this study are somewhat disturbing in that 22% of the time they were wrong about the presence of a pulse. In this study they were given as long as they wanted and in no way were under stress to perform. They simply had to say after taking as much time as they needed whether the pulse was present or not. How accurate do you think they would be with a newborn, covered in amniotic fluid and blood with people giving resuscitation orders? Not very accurate I would say.
The NRP program recommends that a rising heart rate is the best indicator of a successful resuscitation. Two hundred years ago a physician brought the stethoscope into our repertoire of tools at our disposal in medicine. Despite all of our focus on non-invasive monitoring during resuscitation, confirmation of a heart rate should only be done by auscultation. Technology serves a useful purpose by providing confirmation of rhythm after hearing the heart beat but should never be used as a substitute for one of the oldest technologies there is.
by All Things Neonatal | Jul 12, 2015 | General Comments, health care, Neonatal, Neonatology, preemie, Prematurity
Living in Canada we are privileged to have a universal health care system. Privileged in the sense that all citizens are entitled to the same level of care regardless of economic circumstance although the monetary costs to the tax payer is another story and forms the basis of most arguments in the US against adopting such a system down south. My goal of this post though is not to enter into a debate about which system is superior but rather speak of the dollars and cents attributable to being born too early or too small.
In the US such measurements are simpler as costs are more easily measured in a private health care system but each health care region in Canada can measure to a certain degree the costs associated with a hospital stay. Certainly the story of Raquena Thomas made this clear to me. In 2007 she was born in Edmonton after her mother left Jamaica for a visit with family in Edmonton. After delivering she was found to have hypoplastic left heart syndrome (HLHS) and went on to have the first stage of the Norwood procedure. What followed was a bill to the mother for $162576 and for commentary on the discussion that ensued about who should pay the bill see the article here. As I was working at the Stollery Children’s Hospital at the time and cared for this infant it was clear to me after this experience that the hospital indeed has a clear method to calculate costs even if we the taxpayer are blind to such calculations.
Now HLHS is a condition that affects very few infants a year in any given province but what about low birth weight and preterm birth? This as we say in Neonatology is our bread and butter. In 2009 Lim et al published data on the Canadian population in attempt to ascertain the health care costs for these groups of patients (CIHI survey: Hospital costs for preterm and small-for-gestational age babies in Canada)
In this period 1 in 7 babies was born either preterm or small for gestational age. If specifically looking at infants < 2500g defined as low birth weight this represented 6% of all babies born. When you factor in that there were 350000 babies born in that year in Canada we are looking at about 21000 babies nationally. 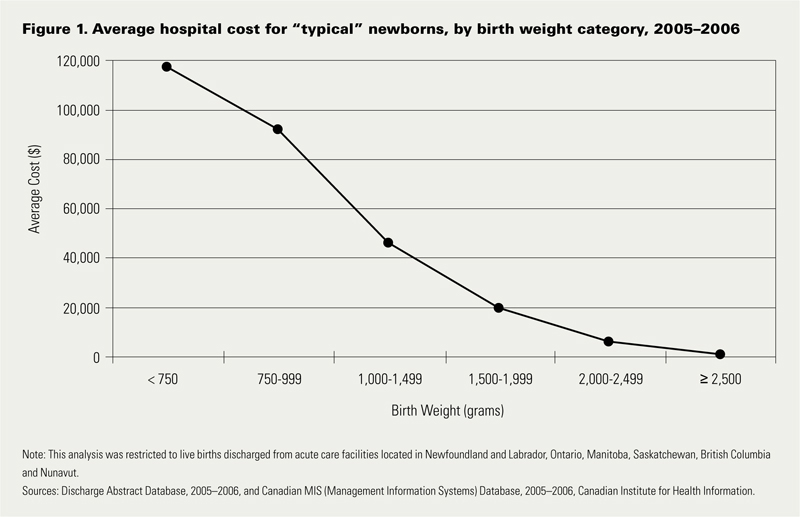 Looking at the costs for these infants one sees a direct relationship between decreasing birth weight and increasing costs in the hospital. This should not be surprising to anyone. It should be noted though that the paper provides average costs only without standard deviation or ranges. As you would expect, the costs for a patient with severe HIE or NEC would be higher than the 26 week infant who has a very smooth course and does not have a symptomatic PDA, severe IVH or any other significant disability during their course.
Looking at the costs for these infants one sees a direct relationship between decreasing birth weight and increasing costs in the hospital. This should not be surprising to anyone. It should be noted though that the paper provides average costs only without standard deviation or ranges. As you would expect, the costs for a patient with severe HIE or NEC would be higher than the 26 week infant who has a very smooth course and does not have a symptomatic PDA, severe IVH or any other significant disability during their course.
The data looking at such costs is scare with respect to the Canadian landscape and even more difficult to determine has been lifetime costs or at least incremental costs after leaving the hospital environment. I was delighted to see that former colleagues of mine in Edmonton have published a new paper examining both the extent of health service utilization (HSU) attributable costs in the year following discharge of both LBW and normal birth weight peers in Alberta (abstract here). Not surprisingly, smaller babies have more medical needs. In this study LBW patients had an average of 5.9 outpatient services and 1.1 visits to the ER in the first year of life compared to 2 and 0.9 in the normal birth weight peers. Physician services were double with 22.7 office visits compared to 11.9 in the NBW group. The costs to the Health Care system overall are represented in the table below which demonstrates that the LBW infants make up 37% of the total health care costs of newborns yet represent only 6% of the population. In terms of risk factors for LBW they were high prepregnancy weight, aboriginal women and low socioeconomic status. Efforts to lessen the incidence of the first and third factor in our pregnant population would be a good target for public health efforts. Bear in mind that the costs outlined below are in addition to the costs in the hospital.
| BW Category |
Cost per patient |
Patients |
Cost to System (millions) |
| NBW |
$3,942 |
43207 |
182 |
| LBW <2500 |
$33,096 |
3123 |
108 |
| 1500 – 2499g (MLBW) |
$20,467 |
2571 |
53 |
| 1000 – 1499g (VLBW) |
$83,895 |
278 |
23 |
| < 1000g (ELBW) |
$117,546 |
274 |
32 |
The analysis provided in this paper does not specify out the costs by certain conditions such as NEC or BPD so all we have to go on are averages for HSU and cost. It does however raise a point which I believe is crucial to any discussions with respect to expanding programs within the hospital. We need to refocus administration at both the hospital level and at the funding source (our provincial governments) as to the true costs of the conditions that we are trying to prevent. It is only through looking at the costs of both the hospitalization and after discharge that we can truly come to understand the cost effectiveness of expanded programs or new treatment modalities.
Donor breast milk is one that I believe serves as a good example of a program that is in need of expansion in many places in the country but is hampered by the perception of high up front costs. The average cost of this milk is about $4 per ounce. I will simplify the math a little as there would be a phase of escalating the volume per day and a wean at the end but let’s say we have a 1.5 kg infant that we want to treat with DBM for a period of 4 weeks. The cost to do this assuming a TFI of 150 mL/kg/d would be a little over $800 per patient so with the increasing phase, wean and adjusting for some weight gain let’s say $1000 per patient. If there were 200 such patients in your hospital each year the annual cost would be $200000 which on the surface seems like a lot of money. From the most recent cochrane review though comparing formula to donor milk the risk ratio to develop NEC is 2.77 meaning that a preterm baby who receives formula is nearly three times as likely to develop NEC. Ignoring differing rates of NEC by hospital let’s just use the concept that we could prevent one case of NEC a year with such a strategy. The cost of medical NEC is somewhere between 100-140K while surgical is 200 – 240K. The in-hospital costs of preventing just one case nearly pay for or exceed the cost of the entire years supply of DBM. If you add to this the cost of the following years of physician visits, consultants, testing, special diets and investigations and procedures these patients receive the costs are more than covered from just one patient.
Health care budgets are no doubt a difficult thing to balance but the point of all of this is that when determining whether to spend our precious health care dollars we must look at not only the impact during the hospitalization but for years after if we truly modify future risks as well.
by All Things Neonatal | Jul 9, 2015 | Neonatology, nutrition
Will that be q2h, q3h or q4h feeding? When I started my residency in Pediatrics that was the question I needed to ask before writing an order to start oral feeding in a preterm infant. At the time it seemed perfectly reasonable but I have to admit the question for me was “What if they aren’t ready?”. Does a baby who won’t take the breast or bottle at the 3 hour mark clearly show they aren’t able to feed or that they really are just not ready to feed? We commonly say that children are not small adults. Hospitalized adults commonly will utter the words “I’m not hungry” when their food tray is brought to them. This may be a reflection of what has been put before them rather than whether hunger exists or not but they seem to be able to be ready to eat so why not children and by extension preterm infants in the NICU.
My approach to feeding premature infants was fairly consistent until about 10 years ago when nurses in Edmonton, Alberta (in a level II unit) introduced me to “semi-demand” feeding. What I find interesting about this, is the paucity of evidence that existed on the subject. At the time, the evidence really centred around one paper but the impact of the approach was undeniable. In 2001 McCain et al published the randomized controlled trial involving 81 infants A feeding protocol for healthy preterm infants shortens time to oral feeding. The concept of semi-demand feeding was to assess each infant (once preterms reached 32-34 weeks CGA) before a feed for signs “of feeding readiness”. This was accomplished through offering non-nutritive sucking every three hours before a scheduled feed. If the infant was found to be in a wakeful state, the oral feeding was commenced but otherwise the infant was left for 30 minutes with NNS attempted again. If the infant was still not ready then a gavage would be given. The key here is that the infants were monitored for signs of feeding readiness rather than insisting upon an arbitrary time for their next feed. The study findings were a halving of the time it took to reach full feeds (10 days in control vs. 5 days in semi-demand) with no difference in weight gain observed between groups. The latter point is worth emphasizing, as the concern with semi-demand has been from some that in a worst case scenario where feeds took place every 3.5 hours a baby would miss one feed compared to another infant on a q3h schedule. This fear though does not bear out in the study.
The experience in the centre I currently work at has been so positive that it is hard to find a patient that is not fed in such a way whether a physician orders the approach or not! What is truly fascinating to me is how effective the approach seemingly is and has been adopted again with very little evidence compared to that traditionally needed to change a practice in the neonatal world. Interestingly, although we can’t say for sure we have noticed year over year declines in length of stay for infants born with a birthweight of 1500 – 2000g since the introduction of semi-demand feeding. This could be a coincidence as this has not been the only practice change in our units but it certainly is interesting.
I was delighted to see a paper published this week on the topic by Wellington and Perlman. This was a Quality Improvement project entitled Infant Driven Feeding in Premature Infants: A Quality Improvement Project. This study compared three periods. The first was one in which physicians set the feeding schedule (PDF), the second a training period for a new system and the last the infant driven period (IDP). In the PDF phase, the physicians would order one oral feed a day, then two, three and so on when the full feed was attained at each prescribed level. In the IDF period an assessment sheet for feeding readiness would be completed before each attempt and the decision to offer an oral feed based on the perceived ability to feed at that time.
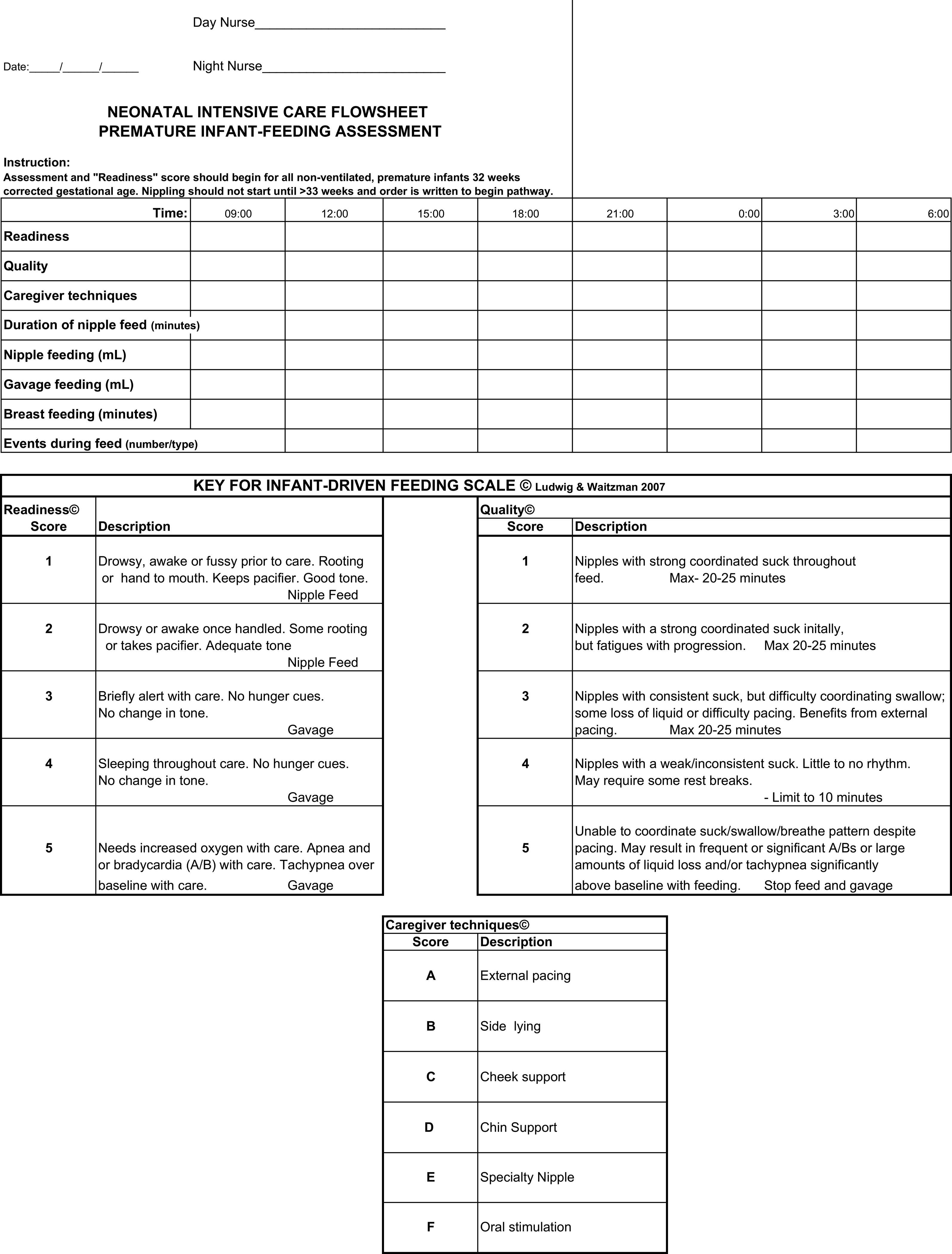
While this study was not an RCT it is a much larger group of patients than the study by McCain. This comparison was between 153 PDF vs 101 IDF patients. Feeding readiness assessments would start at 32 weeks CGA but feedings would not be offered by either approach until 33 weeks CGA similar to our own approach to feeding for the most part. The use of IDF made no difference to timing of first attempt at nipple feeding. The time to attain full nipple feeding was where significant differences in approach became apparent.
Time to reach full nipple feeding by gestational age at birth:
<28 weeks: IDF versus PDF group reached full NF 17 days sooner (374/7 vs 40 weeks; p=0.03)
28–316/7: IDF versus PDF group reached full NF 11 days sooner (35 4/7 vs 37 1/7 weeks; p<0.001)
≥32 weeks: IDF versus PDF group reached full NF 3 days sooner (354/7 vs 351/7 weeks; p=0.04).
Affect on discharge
<28 weeks GA, no difference between the IDF versus PDF group (41 3/7 vs 39 4/7 weeks; p=0.10).
28–316/7 weeks GA, IDF group were discharged 9 days earlier (366/7 vs 381/7 weeks; p<0.001).
≥32 weeks GA, the IDF group were discharged 3 days earlier (36 weeks vs 363/7 weeks;
p=0.048)
Although the findings are clear there does need to be the usual acknowledgement that this is not the gold standard RCT but the practice change in the unit was done pretty carefully. The concept is one that makes a great deal of sense regardless. The lack of difference in discharge for the smallest infants makes some sense as it may well be apnea of prematurity that is the last to resolve. There is no disputing however the benefit in earlier discharge for the 28 – 31 6/7 week group. They achieve feeding earlier and go home faster. From a family centred approach this is the best of both worlds. One should not write off the use of this technique in the smallest infants either as they will have their care normalized much earlier with the NG tube being removed and the parents getting to participate and practice feeding much earlier in their course. Although not measured in this study, it would be intriguing to look at the number of patients who were admitted to hospital post discharge with failure to thrive.
Imagine the impact as well on hospital length of stay data if you multiple the reductions in length of stay by the total number of patients seen in these gestational age categories each year. This almost certainly can represent over a year of patient days for many hospitals.
As I see it the direction is clear. We should not force our premature infants to follow a schedule that works for us. Rather use the cues that only they can provide to tell us when and how much milk they desire. Both the parents, infants and our hospitals will benefit.



 Interestingly one can also see that after 40 weeks these complications rose again. Post term deliveries are not without their consequences either.
Interestingly one can also see that after 40 weeks these complications rose again. Post term deliveries are not without their consequences either.






 Looking at the costs for these infants one sees a direct relationship between decreasing birth weight and increasing costs in the hospital. This should not be surprising to anyone. It should be noted though that the paper provides average costs only without standard deviation or ranges. As you would expect, the costs for a patient with severe HIE or NEC would be higher than the 26 week infant who has a very smooth course and does not have a symptomatic PDA, severe IVH or any other significant disability during their course.
Looking at the costs for these infants one sees a direct relationship between decreasing birth weight and increasing costs in the hospital. This should not be surprising to anyone. It should be noted though that the paper provides average costs only without standard deviation or ranges. As you would expect, the costs for a patient with severe HIE or NEC would be higher than the 26 week infant who has a very smooth course and does not have a symptomatic PDA, severe IVH or any other significant disability during their course.
