by All Things Neonatal | May 30, 2015 | bioethics, Neonatal, Pregnancy
A couple months ago on my Facebook Page (www.facebook.com/AllThingsNeonatal) I posted the shocking story of a 65 Grandmother Annegret Raunigk who received IVF and was carrying quadruplets. The post spawned outrage among my followers with statements that it was simply wrong while others argued that the risks of the mother giving birth to premature infants who would need extensive support was extremely high and that it was unethical to have done so. From my standpoint I agreed with the comments and so here you have it; the mother (or grandmother) has given birth.
http://www.cnn.com/2015/05/24/europe/germany-grandmother-quadruplets/index.html
She has delivered four premature infants < 26 weeks gestational age. Assuming they are 25 weeks each the outcomes for these babies may not be as dire as one might think. We would expect for singletons about a 70 – 80% survival with about 50% surviving without moderate or severe impairment. With quadruplets I would expect lower numbers so the reality is that at least two of these kids will have significant health care needs in the future. I would ask that we leave aside the arguments that may ensue at this point by bioethical purists who may argue that the babies’ perception of their quality of life in adolescence would be better that ours. The reality is that even if this were so, this single mother has now given birth to 17 children of which her last four are extremely low birth weight.
Who will be there to care for these children? What about the impact on society? in the next ten years will she have the energy to provide the stimulation, take the kids to all their appointments and so forth that will be needed to ensure an optimal outcome for them from a developmental standpoint?!
This is wrong on so many levels and it is not enough to say as they mention in the article that doctors encouraged her to undergo fetal reduction by one or two fetuses at an early stage. That excuses the IVF treatment in the first place by ignoring the fact that the opportunity to do something about this was missed at the first doctor’s appointment when she asked for the IVF treatment.
Nadya Suleman the “Octomom” give birth to eight premature infants and became known the world over in a sensational news story that followed her through her pregnancy and birth. It is another example of the medical community embarking on a path that lacks responsibility and ignores one of the founding principals of our Hippocratic Oath “To do no harm”. Nadya’s physician in her case suffered one of the most appropriate punishments that California could dole out. He lost his license (http://nydn.us/1d5rSxc), she went on to file bankruptcy, spoke out against the kids she wanted so much and finally resorted to pornography to pay the bills (http://huff.to/1AE5HsR). I can only wonder if the mainstream media’s obsession with Nadya’s story sparked desires for copycat sensational pregnancy stories elsewhere. Annegret Raunigk will not doubt receive a tremendous amount of attention as she already has so is there another agenda over and above to have children that is at work here?
As a medical practitioner it is generally ill advised by the local Colleges to speak out publicly against another physician but in this case this doctor who chose to implant embryos into the 65 year old mother should suffer the same fate and lose his license before he does such a thing again. He lacked judgement and any sensibility in creating a situation that would almost certainly lead to four extremely premature infants being born. I hope the German medical community acts to remove such a physician (I use the term lightly) before he can cause any further damage.
by All Things Neonatal | May 28, 2015 | Innovation, Neonatal, Neonatology
Update
Based on what you will read below our centre implemented the practice change below. In a two year span we saw our repeat sampling for metabolic screens drop from about 800 per year to 300! A small change with large benefits!
A Little Less Pain
The 1960s saw the emergence of newborn screening for phenylketonuria. This was an important milestone in the field of newborn care as it allowed us to screen children for something that we could do something about. Dietary manipulation could for the first time prevent the repercussions of this condition and allow these children to avoid the severe neurological impairment that would follow the natural course of the condition. Since that time, our ability to screen for and offer treatment to modify other disease courses has expanded many fold which no doubt in terms of population health is a wonderful thing.
Here in Manitoba we are now screening for over 40 conditions with a useful site for information being provided by Manitoba Health.
The expansion of these programs has been possible due to the use of Tandem Mass Spectrometry. This technique provides the ability to screen for many conditions without increasing the amount of blood required.
The downside to more screening is that as the number of tests being sampled increases the risk of false positive results due to the presence of dietary additives. An example of this is carnitine supplementation. In our centre we were providing this to low birth weight infants based on demonstrated low levels of carnitine facilitated lipid metabolism. After failing to find a clinical benefit after the metabolic derangements were noted we identified a larger issue in that many of our premature infants receiving carnitine supplementation had elevated acylcarnitine profiles on their Newborn Metabolic Screening (NMS) samples. These false positive results led to repeated sampling via bloodspot analysis leading to unnecessary blood sampling and pain from heel lances.
Another set of conditions that we are now able to screen for are the aminoacidopathies. This group of disorders involve abnormalities of amino acid metabolism leading to toxic elevations of one or more amino acids that can have significant neurodevelopmental impairment as a consequence. Clearly in all of these tests the purpose is to avoid long-term deleterious consequences but as with carnitine, false positive results are very concerning as they lead to repeated sampling, and potentially larger blood draws if confirmation of the screening results are needed. Add to this, that this further analysis requires consultation with metabolics consultants, nursing time for repeated sampling, and laboratory costs and you can see why minimizing false positives is needed. Lastly the greatest impact is on the family who in many cases experience unneeded anxiety as they await confirmatory testing which may take a week or more to come back if the sample needs to be sent offsite.
A few years back I attended the PAS meeting in Boston and heard about a study on this subject from California that they were presenting in abstract form. Withholding TPN and using D10W for a three hour period prior to collection of the NMS could reduce false positive aminoacidopathy screens by about 70%. The reaction of our local laboratory was one of disbelief as the consensus was that such a short time frame could not clear the TPN sufficiently from the circulation. Since the reference ranges for normal amino acid profiles in infants are from patients who are not receiving TPN this could create false positive elevations, which would require either repeat blood spot sampling or as above, trigger a formal consult to metabolics if the subsequent test is also positive.
In following up on the original abstract presentation I noted that the findings were in fact published as Reduction in Newborn Screening Metabolic False- Positive Results Following a New Collection Protocol.
In this 2 year retrospective cohort study, in 2010 NMS was done for all infants between 24-48 hours with no withholding of TPN and in 2011 the protocol was changed to hold TPN for 3 hours and use D10W before collection of the NMS. The main results of the study are shown below and of note all the False Positive results post intervention were statistically different to a significant degree after the change in practice. Examining the entire group there was a 74% reduction in false positive results post practice change.
|
Preintervention (N=274) |
Post Intervention (N=265) |
| Birth Weight |
Negative (%) |
False Positive (%) |
Negative (%) |
False Positive (%) |
| <1000g |
13 (65) |
7 (35) |
14 (82.4) |
3 (17.6) |
| 1001-1500 g |
23 (92) |
2 (8) |
24 (96) |
1 (4) |
| >1500 g |
222 (96.9) |
7 (3.06) |
223 (100) |
0 (0) |
| Total |
258 (94.2) |
16 (5.83) |
261 (98.5) |
4 (1.50) |
Furthermore an 81% savings in health care costs per patient were realized in the change as well. This is outlined below:
| Item |
Preintervention |
Postintervention |
| Confirmatory testing |
4653 |
434 |
| Supplies for testing |
46 |
12 |
| Supplies for new protocol |
0 |
465 |
| Total |
4699 |
911 |
The results speak for themselves yet the practice I don’t believe has been widely adopted and certainly not in our centre. This past week however the following study was released in abstract form and inspired me to write this post as I believe the evidence is overwhelmingly in support of this practice change Stopping Parenteral Nutrition for Three Hours Reduces False Positives In Newborn Screening.
12 567 consecutive births in 1 hospital between May 2010 and June 2013 were analyzed to determine the FP for AA levels in the NMS. The FP rate in infants > 1500g was much lower overall than for those under 1500g which may have been explained by less TPN use in that cohort. Similar to the first study TPN was changed to D10W for three hours prior to collection of the NMS and resulted in a FP rate of 3.1% in the D10W group vs 11.8% in the TPN group. This represents again an overall 74% reduction.
So there you have it. Two studies showing the same results. The concept is simple, saves hospitals money and more importantly avoids unnecessary parental anxiety, needless blood sampling and consumption of time by nursing staff and other consultants. This is not a high tech strategy that takes a great deal of education to implement. Rather this can be started tomorrow wherever you are and it is my hope that by reading this at least one hospital out there aside from our own may adopt this small change to make a big impact on our patients.

by All Things Neonatal | May 15, 2015 | caffeine, Neonatal, Neonatology
For those of you who know me and my practice as a Neonatologist you may find the title of this piece odd. I have and will likely continue to be an advocate for the use of caffeine in premature infants. I recommend it both very early in the caseroom for those under 32 weeks to help stave off intubation and often continue caffeine until late in an infants’ stay in the NICU. Truth be told I also send children home on caffeine on occasion when all other markers needed for discharge have been met but they continue to have episodes of apnea and bradycardia that are not resolving and prolonging their stay in hospital.
In recent years I have noticed a creep of practice to begin pushing doses of caffeine base beyond the 5 mg/kg level that has been generally accepted as the upper limit of the 2.5 to 5 mg/kg range that most use in practice. The standard dosing was justified based on the CAP study by Schmidt et al indicating that it was effective in reducing the risk of bronchopulmonary dysplasia and success at earlier extubation. While there appeared to be an initial benefit to neurodevelopment favouring caffeine treatment by school age the difference disappeared. This creep effect to using higher daily maintenance dosing of 7 or 8 mg/kg/d has occurred likely for some good reasons not the least of which is a dose effect in which clinicians could see a reduction in clinical events for some patients as they increased the dose. We are no different as doctors than others in that success tends to shape our practice. Now before you accuse us of being mavericks, we did have some evidence to support the use of higher dosing beyond the 5 mg/kg dosing that had been recommended. Published in 2004, Steer and colleagues studied the effect of using a loading dose of 80 mg/kg caffeine citrate (take 50% reduction to get the base formulation we normally use) followed by 20 mg/kg maintenance dosing vs 20 mg/kg loads and 5 mg/kg maintenance in a cohort of infants < 30 weeks gestation who were having a planned extubation. The full article may be found here. The results of the study demonstrated greater success in extubation and less apnea in the group treated with the higher doses as shown here.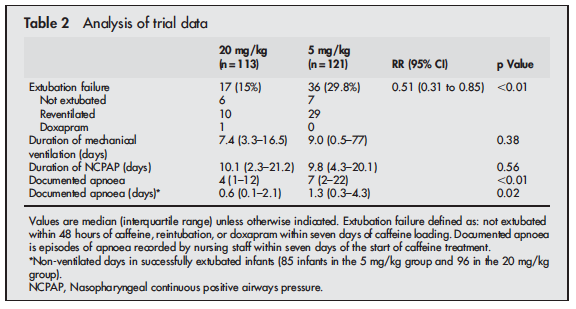
The results of this study certainly made some waves in the Neonatal community as can be seen by the “creep” in practice over the last number of years to increase the caffeine dose in our units to 6, 7 and sometimes 8 mg/kg of caffeine base in an effort to essentially titrate to effect especially in infants who are on CPAP. The motivation to prevent a reintubation secondary to apnea has been so compelling that the theoretical concerns over lack of long-term outcome data on high dose caffeine treatment have been largely ignored.
At this point it is important to also recognize that the way in which we use caffeine in terms of initiation of treatment has also changed. Many units have adopted the “Golden Hour” approach to neonatal resuscitation and are driven to use non-invasive means of support after encouraging results from several trials such as the Support, Boost and the more recent Canadian NIPPV trial. While not demonstrating improvements in outcomes necessarily, the fact that BPD rates are mostly unchanged means that with the use of early caffeine in the delivery room and the use of CPAP one can avoid invasive ventilation in many infants. As such, there has been a departure from the practice as described by Steer and colleagues to using caffeine to facilitate extubation to trying to prevent it in the first place.
In discussions with some of my colleagues we have expressed some reservation over the use of the higher doses of caffeine beyond 5 mg/kg and with the publication of a study this week by McPherson et al, these concerns may be quite warranted. For the complete study click here. This study of 74 preterm infants randomized them in the first 24 hours of life to either 80 mg/kg or 20 mg/kg caffeine citrate loads and then in both groups they followed these loads with 10 mg/kg per day maintenance. The primary outcome of the study was white matter structural development by MRI. Previous research by Doyle had found an improvement in this outcome with the use of standard caffeine therapy of 10 mg/kg/d so the real question here was “If a little is good, then is more better?”
Sadly the answer to the last question is a resounding NO!
None of the respiratory outcomes were any different between the standard caffeine and high dose groups but the following came out as a worrisome outcome:
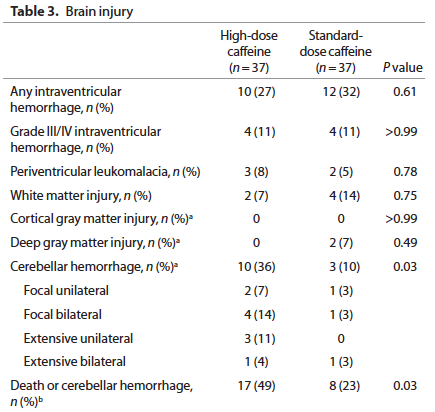
Furthermore when the infants were followed up at 2 years of age a statistically significant percentage of 2 year olds previously randomized to the high dose caffeine regimen were found to be hypertonic (2.3 vs. 1.5%). Overall neurodevelopment was no different between groups but it should be pointed out that the study was not powered to detect such differences.
One question that must come up with these findings is whether or not it is plausible that a 2 day exposure to high dose caffeine followed by standard dosing for the remainder of the time could lead to cerebellar hemorrhage. I think the answer is yes given the findings from a single dose of 25 mg/kg caffeine (equivalent to 50 mg of caffeine citrate/kg as studied by Hoecker et al
http://www.ncbi.nlm.nih.gov/pubmed/11986437
As noted by the authors, this single dose was responsible for reducing cerebral blood flow velocity by about 20% from baseline. The regimen over 48 hours in the above study was to give 80 mg/kg in divided doses as a load so it is reasonable to conclude these infants would have experienced a reduction in cerebral blood flow as well, and possibly to a greater degree than the patients in the Hoecker study. Add to this that these are infants under 30 weeks of age who have a fragile arterial and venous network to begin with and it seems reasonable that a period of hypoperfusion possibly combined with hypoxemia and then reperfusion injury could account for these cerebellar bleeds.
So where does this leave us? As the authors conclude it is not wise to plan a larger study looking at the same strategy given the findings in this pilot. What remains unclear at least to me is whether 6, 7 or 8 mg/kg during the maintenance phase of treatment offers any true long-term advantage. With anything there are tradeoffs though and finding the right balance is never easy. If we use lower caffeine doses and in some patients they require intubation, is the increased risk of CLD and possible neurodevelopmental impairment from that worth the limitation of risk? After the first week of life is the risk of cerebellar hemorrhage lower as the blood vessels mature? I think so which would make the argument for using higher doses at that point but in truth we just don’t know about safety in terms of long-term outcomes. For now at least it would seem that in the absence of guidance from research all we can really say is that 2.5 to 5 mg/kg/d of caffeine base is safe but that doses higher than that need to be used with caution. It may be wise to seek informed consent for the use of higher doses in light of these findings but it is up to each unit to decide if this is justified based on your views of the data. What do you think?
by All Things Neonatal | Apr 27, 2015 | hypoglycemia, Neonatal
I was inspired to write this post after sharing a review of an article from 2013 on my Facebook page. The article pertained to the use of a 40% dextrose gel to treat neonatal hypoglycemia
We have been using this glucose gel in our population for nearly two years and have noted great success in avoiding admissions for hypoglycemia, however this remains unpublished. I was surprised to hear how many places have yet to adopt such treatment and based on the comments on the page it would appear that adoption of such gels are on their way in some locations. The popularity of this post though inspired me to write this piece, which summarizes the evidence for the use of gels in the neonate.
What is the Evidence For Using Glucose Gels
Surprisingly there is actually very little in the way of publications on the topic. In 1992, there was a small randomized trial which failed to show a benefit in terms of variability of one serum glucose to the next but it did not look at other functional outcomes such as impact on maternal infant separation or success in breast-feeding.
The next study is in fact the one mentioned in the article that was posted on Facebook called the Sugar Babies study. Dr. Harris in this case studied 118 infants who received 40% dextrose gel vs 119 who received a placebo gel. All of the infants in this study were selected based on risk factors for hypoglycemia (IDM, IUGR, LBW, LGA, near term) and were all 35 weeks or greater. Each infant had to be less than 48 hours of age when enrolled. Infants received 0.5 mL/kg 40% dextrose gel (200 mg/kg). This was designed to deliver the same amount of sugar as would be given with a D10W bolus of 2 mL/kg. In order to receive the treatment the blood glucose had to be < 2.6 mmol/L so equivalent to our own standards in Canada and the US. Treatment failure, which was the primary outcome was defined as a blood glucose < 2.6 mmol/L despite two treatments with gel. The significant findings were quite interesting and are shown in the table below.
| Finding |
Dextrose |
Placebo |
| Treatment Failure |
14% |
24% |
| Admission to NICU |
14% |
25% |
| # formula feeds (median) |
7 |
10 |
| Formula fed at 2 weeks |
4% |
13% |
What was not found to be significant and in and of itself is a very important finding is a higher incidence of rebound hypoglycemia in the dextrose gel group. This was a potential concern as provision of dextrose in theory could cause a spike in insulin secretion thereby dramatically lowering the blood glucose but thankfully this was not observed.
Dextrose Gel Improves Breastfeeding Rates
These results I believe speak for themselves but it is extremely important to highlight the benefit here. The use of the dextrose gel was also able to enhance success at breastfeeding rates. This was accomplished in all likelihood by a reduction in admission to NICU and less reliance on formula to achieve satisfactory blood glucose. As these infants were all less than 48 hours old it is safe to assume that in many cases the mother’s milk had not yet come in so if the glucose measured was low, health care providers were more likely to intervene with an offering of formula. It is worth noting that while this is the only significant study in the field there is a letter to the editor in which another author describes the use of a sublingual sugar powder for treating the same, which was met with similar success. There is no actual peer-reviewed study to examine however so we will leave it as simply an interesting point.
New Study on The Way
If these results leave you still being skeptical you may be pleased to hear there is a very large study (2129 babies needed) beginning enrolment in New Zealand with the primary outcome of admission to the NICU. This prospective RCT will hopefully put to rest any questions about this treatment that have delayed implementation in many units.
As a final thought regarding the Sugar Babies study, one of the differences that came close to reaching statistical significance was the rate of IV insertions for dextrose. In the dextrose gel group the rate was 7% vs 14% in the placebo. With a p value of 0.09 it suggests that with a larger study size a difference may have been reached. The idea that we have the option of using a therapy that can decrease formula use, improve breastfeeding rates including those found post discharge and lastly decrease the poking of infants for IV dextrose is a goal well worth pursuing. Is this enough evidence for you? I would encourage all who read this piece to ask their NICU the question of whether a trial of dextrose gel is worthwhile. It could make a big difference far beyond treating a number.
by All Things Neonatal | Apr 13, 2015 | minimizing bloodwork, Neonatal, Pain in the Neonate
As those of you who have been following this blog are aware, I am always on the lookout for strategies that can help minimize blood work without sacrificing care in the NICU. At particular risk our the very premature infants in our units who for example at 1 kg have about 80-90 mL of blood. It does not take very many 0.5 – 1 mL “small” draws to create anemia. In a recent study (free article in link) of infants less than 1500g entitled A mathematical modeling approach to quantify the role of phlebotomy losses and need for transfusions in neonatal anemia, the authors studied 26 infants over a one month period. The results were staggering in that these infants experienced 138 +/- 21 blood draws with an average of nearly four transfusions per patient. While the authors do not specify what type of testing was done they did find a shocking statistic that 59% of the blood collected by weight of sample was discarded. This certainly stresses the point that we should aim to minimize the volume of sample collected in each case to that which is only necessary for the equipment to run. Furthermore, strategies to minimize sample draws should be utilized where possible and if accuracy permits point of care technology may further reduce volumes required and provide immediate results at the bedside. Lastly where possible, utilizing non-invasive technology to avoid blood draws needs to be explored when possible and was the subject of another post on Masimo non-invasive HgB measurement (http://wp.me/p5NWfD-1t).
Certainly in sick neonates whether they be term or preterm the drawing of blood gases to monitor ventilation contributes to the anemia of prematurity which often culminates in a transfusion. Sicker infants with greater lability due to respiratory compromise are deserving of optimal ventilation and this is achieved by monitoring pCO2 levels in arterial or venous samples. There have been different strategies employed to replace the sampling of CO2 by blood gas analysis which have not been very successful but there is one that I believe has promise that I will discuss at the end.
Transcutaneous pCO2 measurement was introduced in the 1980s. While this technology does allow measurement of pCO2 the variation between true arterial pCO2 and tcPCO2 can be wide making the technology difficult to implement on a consistent basis. In particular the accuracy in infants <28 weeks has been quite poor leading to increased numbers of arterial and venous samples to “check” ow closely the results correlate. As was described in 2005 by Aliwalas LL et al the technology in this group who actually have the highest number of blood draws does not meet the required standard to replace arterial pCO2 measurements (http://www.ncbi.nlm.nih.gov/pubmed/15496874)
Another method is of directly sampling exhaled CO2 in ventilated patients. Traditionally such measurements were taken with proximal gas sampling and in neonates in particular the results were discouraging. Problems encountered with proximal end tidal sampling were related to the lack of cuffed endotracheal tubes in part as the measured gas would be diluted with air in the presence of any leak around the tube leading to underestimation of true CO2 levels. Furthermore, in the presence of significant pulmonary disease the clearance of CO2 may be impaired such that the arterial pCO2 – ETCO2 difference may be quite large. For a review see the free article by Malloy and Deakins Are carbon dioxide detectors useful in neonates? The agreement between arterial and proximal sampling measured in this way has been quite variable and as such the technology has not really caught on to any great degree for monitoring ventilated infants. That being said it can be quite useful at determining if the endotracheal tube is in the trachea or esophagus. The presence of the waveform even if not yielding an accurate level confirms proper placement although where the tube sits in the trachea still needs confirmation.
The final method for sampling CO2 is the one which I believe holds the most promise for actually reducing blood draws and by extension risk of anemia and pain in the neonate. Kugelman and colleagues in Haifa, Israel published the following paper (free article in the link) A novel method of distal end-tidal CO2 capnography in intubated infants- comparison with arterial CO2 and with proximal mainstream end-tidal CO2. This creative study utilized a double lumen endotracheal tube which had been designed for surfactant installation and distal pressure measurement to instead sample pCO2 near the carina. This strategy was postulated to eliminate the issue with dilution of gas from proximal sampling and provide a closer measurement of true pCO2 when compared to arterial CO2 and proximal sampling. They studied 27 infants with varying degrees of pulmonary condition severity although most had RDS. When comparing the three methods of pCO2 measurement the following was found.
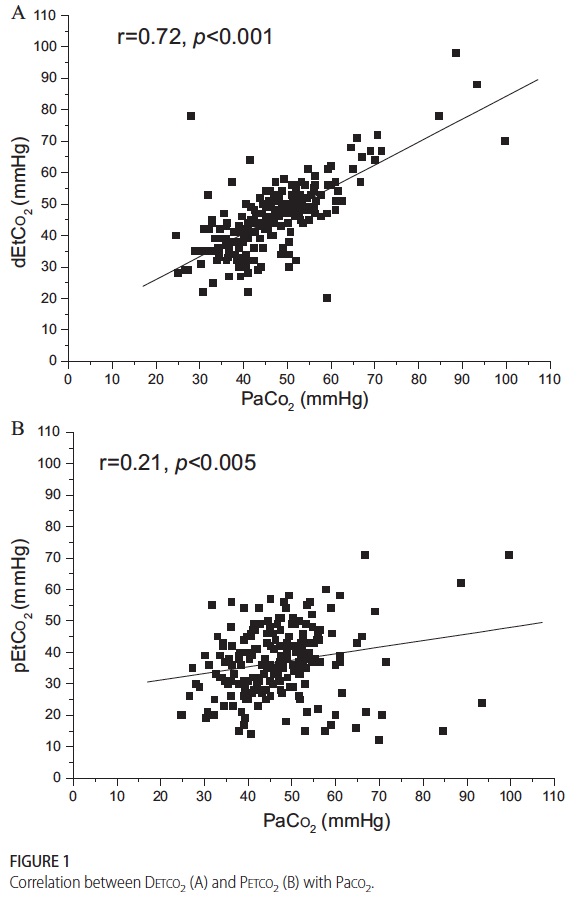 This demonstrates that while proximal measurement was quite poorly correlated with true arterial pCO2 the distal measurement was much more accurate. In fact the mean differences between arterial pCO2 and distal measurement was -1.5 mm Hg while that of proximal measurement -10.2 mm Hg albeit with wide confidence intervals. As found in other studies of proximal end tidal CO2 measurement, worse pulmonary disease correlated with worse accuracy as shown in table 2.
This demonstrates that while proximal measurement was quite poorly correlated with true arterial pCO2 the distal measurement was much more accurate. In fact the mean differences between arterial pCO2 and distal measurement was -1.5 mm Hg while that of proximal measurement -10.2 mm Hg albeit with wide confidence intervals. As found in other studies of proximal end tidal CO2 measurement, worse pulmonary disease correlated with worse accuracy as shown in table 2.
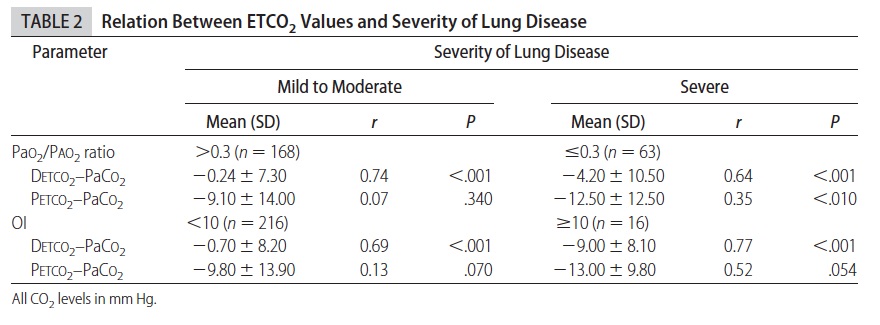
As the pCO2 rises above 60 the accuracy is less but remains much better than proximal measurements. Interestingly the same group has published an additional trial using high frequency ventilation and confirmed the measurements remain accurate. (http://www.ncbi.nlm.nih.gov/pubmed/22328495)
So what does the future hold? in VLBW infants one concern may be the internal diameter of the smallest double lumen tubes and the effect of upsizing to a larger tube and risk of subglottic stenosis. After a personal communication with Dr. Kugelman I understand that this has not been an issue in their unit as they tend to use these double lumen tubes in most if not all of the their infants. The accuracy is sufficient enough from my point of view that units should be able to implement this strategy at least in larger infants at first (those who would need a 3.0 ETT and larger) to see the effect on blood sampling. I suspect that one blood gas a day to determine accuracy in a given patient would be sufficient most of the time if the numbers were found to correlate well.
I would welcome feedback from people who work in units where this strategy has been utilized. How effective is it? Did it reduce your blood gas draws or increase them due to unreliability? Have you seen a rise in subglottic stenosis? Please send your feedback to either this site or at my Facebook page at www.facebook.com/AllThingsNeonatal.
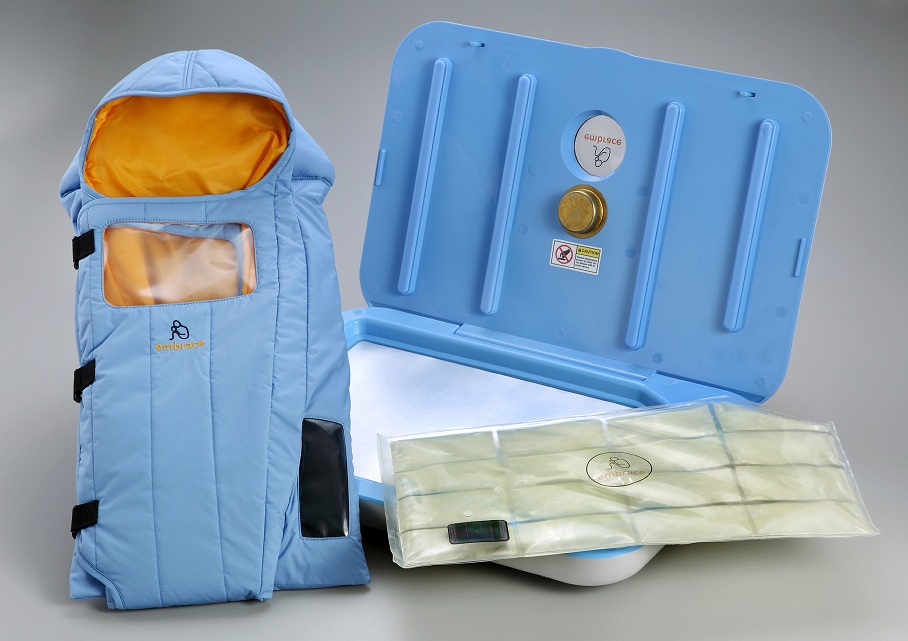
by All Things Neonatal | Mar 27, 2015 | Neonatal, Prematurity, Transport
In the modern day NICU thermoregulation is something that we are concerned with but due to the availability of servo controlled warmers in delivery suites hypothermia is becoming a rarer event. Add to that we have access to polyurethane plastic wraps for our smallest infants and admission into servo controlled environments with additional humidity control and we have all the tools available to prevent hypothermia and it’s consequences. Such adverse effects include hypoglycemia and lethargy the latter being a common cause of septic work-ups leading to increased antibiotic usage. Such interventions are both painful and put the infant at risk of complications related to antibiotic use such as increased resistance, or altered microbiome with puts them at an increased risk of NEC. Just published online today in fact, researchers from the Canadian Neonatal Network have shown a U shaped relationship between admission temperature and outcome (morbidity and mortality including ROP, BPD and NEC etc) from being hypo or hyperthermic on admission. http://bit.ly/1H2384x. Clearly there is harm from being hypothermic so maintaining normothermia must be of prime importance in the care of our patients.
Sadly such technology does not exist everywhere and in developing nations the common alternatives may be non-servo controlled warmers, warmed blankets, cribs or Kangaroo Care (Skin to Skin). There is no question that Skin to Skin (STS) care provides excellent thermoregulation and bonding for the parent and child but what do you do when the mother or father is unavailable (ill after delivery) or unwilling? The benefits of STS to prevent hypothermia and infant mortality are striking and were first described in 1978 by Drs Rey and Martinez. A brief history of the practice following their description can be found here http://bit.ly/1CgDm9t.
This month Bhat SR et al published the following article (http://1.usa.gov/1HRRZoo) Keeping babies warm: a non-inferiority trial of a conductive thermal mattress. Arch Dis Child Fetal Neonatal Ed. 2015 Mar 19
This study compared the above mentioned modes of warming infants with a warming mattress over a four hour period in a non-inferiority trial of temperature regulation. The concept here is that the authors were simply trying to show that the use of the mattress as a thermoregulation device was just as good as the other means of controlling temperature. For a video demonstrating this technology please look at http://embraceglobal.org/. Briefly the system involved a containment device that has a paraffin based material that when preheated with an inexpensive electric heater absorbs the heat and then when placed in a pouch provides constant temperature regulation for a 4-6 hour period. The pouch was subsequently placed in a mattress with wrap as shown in the video to yield the desired temperature regulation. The authors of this study looked at a total of 160 infants with a birthweight between 1500 – 2499g in four centres located in India and observed infant temperatures hourly over a four hour period. Temperatures were compared in each case between those randomized to the standard temperature regulation strategies in each centre versus the warming mattress. The findings were in agreement with the hypothesis of the study in that the mattress was equivalent to the other methods of regulating temperature.
Specifically the temperature of the infants during the 4 hour trial period was 0.11 +/1 0.03 degrees higher than the other methods. None of the infants developed hypothermia during the trial and while 5 of the infants randomized to the mattress were withdrawn from the study it was due to the temperature of the infants being in the upper range of an acceptable level between 37.5 – 37.9 degrees.
My first reaction when I read this paper was that it was interesting but did it really apply to our population? As the authors suggest it was not a blinded intervention and over time, temperature regulation improved so could the Hawthorne effect be at play? The infants in this study were larger (1500 – 2499g) than the infants that we would typically put at highest risk. Also how useful is an intervention that only lasts 4-6 hours when we need to care for these kids for weeks? Lastly, I live in North America and work in an intensive care unit with access to state of the art equipment for thermoregulation and also have a team that proudly promotes STS care so is this really needed? Despite all of these concerns the conclusion I have come to is that the technology provides reasonable thermoregulation for a 4-6 hour period. Can this be applied to our patients in the end? I think there may be a role.
I would see the role being in managing newly born infants in remote communities prior to the arrival of a Neonatal Transport Team. In our own centre about 75% of our patients or about 230 patients are transferred from outside of the city borders. On occasion, a premature infant will be born in such places and hypothermia on arrival of the team is not an uncommon occurrence. In Manitoba and many parts of Canada there are communities that are quite isolated from tertiary care centres. These centres have limited equipment and even then it can often be quite outdated if functioning properly at all. Given that the average time to arrival for such infants is less than the 4-6 hours that the mattress provides warmth for, this would seem to be a very beneficial tool to have in such communities. It appears to be the ideal product as the website indicates the following
- Special phase change material in WarmPak maintains a temperature of ~37 °C for at least 4 hours
- Does not require a constant supply of electricity Portable for in-clinic or transport usage
- Reusable and easy to sanitize and reuse
- Enables mother-to-child bonding
As I write this I wonder how many other centres not just in Canada but also in the USA would benefit from looking into such technology. Providing servo controlled infant warmers for each centre that delivers infants is certainly the gold standard and in fact is recommended for all neonates undergoing resuscitation at 10 minutes of age. While ideal we need to acknowledge that some centres do not have such resources so this could very well serve a useful purpose particularly in the Northern US and Canada. According to the Embrace website the concept for this came out of a student project at Stanford University with the goal of designing an infant warmer with a cost of <1% of a traditional infant warmer (about $20000). If the cost is then $200 for the warmer for a completely reusable warming mattress I think they have hit the mark.
Finally, it must be pointed out again that the smallest infants treated in this way have been 1500g. We do not know if smaller infants would remain normothermic or become hypothermic if the same paraffin sized material was utilized. It will be interesting to see if Embrace releases a smaller unit for infants under 1500g. If proven to be successful in maintaining normothermia in this population I believe the use of this device will become widespread. Such a simple concept to treat a big problem in Neonatal Transport!








 This demonstrates that while proximal measurement was quite poorly correlated with true arterial pCO2 the distal measurement was much more accurate. In fact the mean differences between arterial pCO2 and distal measurement was -1.5 mm Hg while that of proximal measurement -10.2 mm Hg albeit with wide confidence intervals. As found in other studies of proximal end tidal CO2 measurement, worse pulmonary disease correlated with worse accuracy as shown in table 2.
This demonstrates that while proximal measurement was quite poorly correlated with true arterial pCO2 the distal measurement was much more accurate. In fact the mean differences between arterial pCO2 and distal measurement was -1.5 mm Hg while that of proximal measurement -10.2 mm Hg albeit with wide confidence intervals. As found in other studies of proximal end tidal CO2 measurement, worse pulmonary disease correlated with worse accuracy as shown in table 2.
