by All Things Neonatal | Jun 5, 2019 | resuscitation
Just about all of our preterm infants born at <29 weeks start life out the same in terms of neurological injury. There are of course some infants who may have suffered ischemic injury in utero or an IVH but most are born with their story yet to be told. I think intuitively we have known for some time that the way we resuscitate matters. Establishing an FRC by inflating the lungs of these infants after delivery is a must but as the saying goes the devil is in the details.
The Edmonton group led by Dr. Schmolzer has had several papers examined in these blogs and on this occasion I am reviewing an important paper that really is a follow-up study to a previous one looking at the impact of high tidal volume delivery after birth. I have written on this previous paper before in It’s possibile! Resuscitation with volume ventilation after delivery. On this occasion the authors have published the following paper; Impact of delivered tidal volume on the occurrence of intraventricular haemorrhage in preterm infants during positive pressure ventilation in the delivery room.This observational study had a simple enough premise. Will the use of Vt > 6 mL/kg in infants given PPV for at least two minutes lead to worse rates of IVH? All infants were < 29 weeks and if they had chest compressions or epinephrine were excluded. All infants were treated equally in terms of delayed cord clamping and antenatal steroid provision. Ventilation was done with a t-piece resuscitator and Vt measured with an NM3 monitor connected to the face mask. First ultrasounds were done for all at 3 days of age.
What did the authors find?
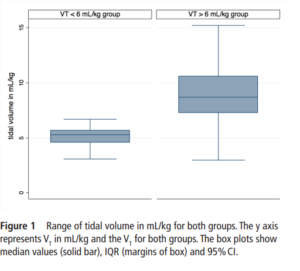
One hundred and sixty five infants comprised this cohort. Overall, 124 (75%) infants were in the high volume group compared to 41 (25%) with a mean VT<6 mL/kg. Median Vt were 5.3 (4.6-5.7) ml/kg for the low group and 8.7
(7.3-10.6) mL/kg which were significantly different.
 When looking at the rates of IVH and the severity of those affected the results are striking.
When looking at the rates of IVH and the severity of those affected the results are striking.
IVH in the high VT group was diagnosed in 63 (51%) infants compared with 5 (13%) infants in the normal VT group (P=0.008).Severe IVH (grade III or IV) developed in 33/124 (27%) infants in the high VT group and 2/41 (6%) in the normal VT group (P=0.01)
Hydrocephalus, following IVH developed in 7/49 (14%) and 2/16 (13%) in the >6 mL/kg and <6 mL/kg VT groups. Looking at other factors that could affect the outcome of interest the authors noted the following physiologic findings. Oxygen saturations were lower in the low volume group at 6, 13 and 14 min after birth while tissue oxygenation as measured by NIRS was similarly lower at 7,8 and 25 min after birth (P<0.001). Conversely, heart rate was significantly lower in the VT>6 mL/kg group at 5, 20 and 25 min after birth (P<0.001). Fraction of inspired oxygen was similar in both groups within the first 30 min. Systolic, diastolic and mean blood pressure was similar between the groups. What these results say to me is that despite having lower oxygen saturations and cerebral oxygen saturation at various time points in the first 25 minutes of life the infants seem to be better off given that HR was lower in those given higher volumes despite similar FiO2. Rates of volume support after admission were slightly higher in the high volume group but inotrope usage appears to be not significantly different. Prophylactic indomethacin was used equally in the two cohorts.
Thoughts for the future
Once a preterm infant is admitted to the NICU we start volume targeted ventilation from the start. In the delivery room we may think that we do the same by putting such infants on a volume guarantee mode after intubation but the period prior to that is generally done with a bag and mask. Whether you use a t-piece resuscitator or an anesthesia bag or even a self inflating bag, you are using a pressure and hoping not to overdistend the alveoli. What I think this study demonstrates similar to the previous work by this group is that there is another way. If we are so concerned about volutrauma in the NICU then why should we feel any differently about the first few minutes of life. Impairment of venous return from the head is likely to account for a higher risk of IVH and while a larger study may be wished for, the results here are fairly dramatic. Turning the question around, one could ask if there is harm in using a volume targeted strategy in the delivery room? I think we would be hard pressed to say that keeping the volumes under 6 mL/kg is a bad idea. The challenge as I see it now is whether we rig up devices to accomplish this or do the large medical equipment providers develop an all in one system to accomplish this? I think the time has come to do so and will be first in line to try it out if there is a possibility to do a trial.

by All Things Neonatal | May 1, 2019 | IVH
Recently the practice of keeping ELBW infants with a midline head position for the first three days of life has been recommended to reduce IVH as part of a bundle in many units. The evidence that this helps to reduce IVH has been somewhat circumstantial thus far. Studies finding that decreased sagittal sinus blood flow, increased cerebral blood volume with increased intracranial pressure all occur after head turns would theoretically increase the risk of IVH. Raising the head of the bed would help in theory with drainage of the venous blood from the head and in fact systemic oxygenation has been shown to improve with such positioning. This presumably is related to increased cardiac output from better systemic venous return.
Bringing it to the bedside
Interestingly, some of the above studies are from over thirty years ago. We now have some evidence to look at involving this practice. Kochan M et al published Elevated midline head positioning of extremely low birth weight infants: effects on cardiopulmonary function and the incidence of
periventricular-intraventricular. The study involved maintaining ELBW infants in an elevated midline head position (ELEV- supine, head of bed elevated 30 degrees, head kept in midline) versus standard head positioning (FLAT–flat supine, head turned 180 degrees every 4 h) during the first 4 days of life to see if this would decrease in the incidence of IVH. Ninety infants were randomized into both arms of the study. In terms of baseline characteristics, BW of 725g in the FLAT vs 739 in ELEV were comparable as well as GA both at 25 weeks. Two differences on the maternal side existed of 40% ELEV vs 24.4% FLAT of mothers having preeclampsia and 23.3% FLAT vs 10% ELEV having prolonged rupture of membranes both of which were statistically significant.
What did they find?
Ultrasounds were performed at entry into the study and then daily for days 1-4 and then on day 7 with abnormal scans repeated weekly. In terms of IVH the authors noted no overall difference in rate of IVH. What they did find however was a statistically significant reduction in the rate of Grade IV IVH.
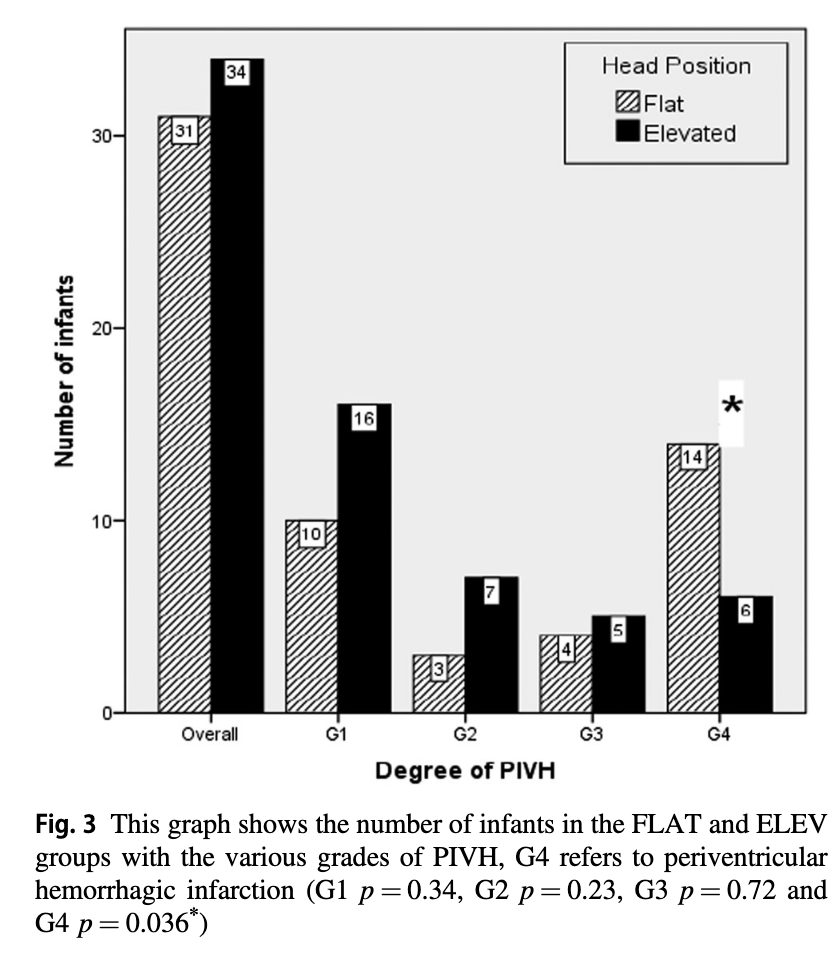
The p value for the finding of lower rates of Grade IV IVH was 0.036 so not strikingly significant but different nonetheless. Given that the venous drainage of the head is also dependent on the resistance to flow from the pressure in the thorax one can’t infer that the intervention alone is responsible for this without ensuring that that respiratory findings are similar as well. Similarly without knowing inflow of blood into the head as measured by blood pressure it is difficult to say that the reduction in IVH isn’t related to differences in blood pressure.
The authors helpfully looked at both of these things. For those infants on high frequency ventilation the mean airway pressure was higher on day one being 11.5 cm H2O (FLAT) vs 9.9 cm H2O (ELEV) neither of which are high although different. The rest of the three days were no different. For those on conventional ventilation the only difference was on day 4 where the MAP was higher for ELEV at 8 vs 7.4 cm H2O which again is fairly mild. Interestingly, as was found in other studies that oxygenation was improved with elevation of the head, the maximum FiO2 for the two groups was different on day 1 being 46% in the FLAT vs 37.5% in the ELEV.
Looking at the hemodynamic side of things there were differences in the lowest mean BP recorded on day 1 and 3 but otherwise the groups were similar.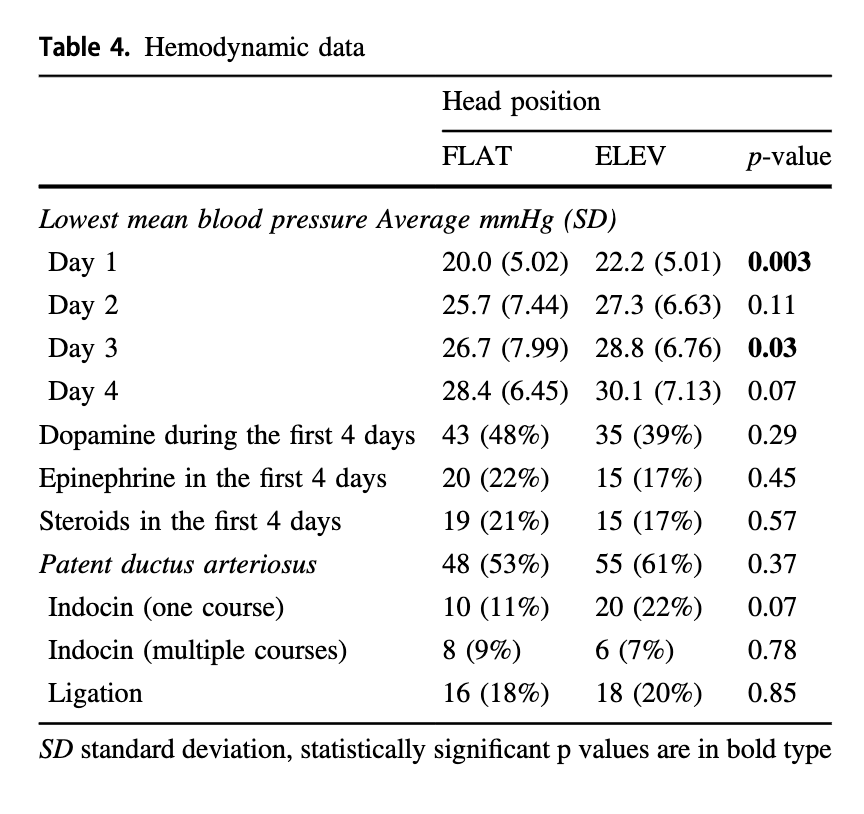 It would have been nice to see mean results during this time rather than lowest but this is what we have.
It would have been nice to see mean results during this time rather than lowest but this is what we have.
In terms of complications of preterm birth there were no differences found in rates of sepsis (important given the increase rate of prolonged rupture in the FLAT group), NEC or ROP.
Although length of stay was no different 92 vs 109 days ELEV (NS), survival to discharge was at 88% vs 76% (p=0.033) which also may explain the longer length of stay.
What Can We Learn From This
Don’t worry. I am not about to throw the results out. There are a couple observations though that need to be addressed. The first is the increased rate of preecampsia in the ELEV group. This finding could have impacted the results. We know that fetuses exposed to this condition are stressed and are often born with better lungs than their non-exposed counterparts. The endogenous increase in steroids due to this stress is attributable and may explain the better oxygenation and lower mean airway pressures needed in the ELEV group rather than improvements in flow alone from positioning. The second issue is adherence to the protocol as there were some infants in the ELEV group who were placed flat for the final 1-2 days of the study. Having said that, this would serve to dilute the effect rather than strengthen it so perhaps it makes the results more believable.
So where does this leave us? This study demonstrates improved survival and a reduction in Grade IV IVH without an overall reduction in IVH. There was nothing found to suggest that the intervention is harmful. Given the background studies demonstrating improved systemic oxygenation, reductions in ICP and cerebral blood volume the finding of reduced severe IVH seems plausible to me. This could be a practice changing study for some units who have perhaps only adopted midline positioning in the first few days of life. It will be interesting to see if this takes off but is certainly worth a good look at.

by All Things Neonatal | Nov 15, 2018 | Breastfeeding
As a Neonatologist, there is no question that I am supportive of breast milk for preterm infants. When I first meet a family I ask the question “are you planning on breastfeeding” and know that other members of our team do the same. Before I get into the rest of this post, I realize that while breast milk may be optimal for these infants there are mother’s who can’t or won’t for a variety of reasons produce enough breast milk for their infants. Fortunately in Manitoba and many other places in the world breast milk banks have been developed to provide donor milk for supporting these families. Avoidance of formula in the early days to weeks of a ELBWs life carries benefits such as a reduction in NEC which is something we all want to see.
Mother’s own milk though is known to have additional benefits compared to donor milk which requires processing and in so doing removes some important qualities. Mother’s own milk contains more immunologic properties than donor including increased amounts of lactoferrin and contains bioactive cells. Growth on donor human milk is also reduced compared to mothers’ own milk and lastly since donor milk is obtained from mothers producing term milk there will be properties that differ from that of mothers producing fresh breast milk in the preterm period. I have no doubt there are many more detailed differences but for basic differences are these and form the basis for what is to come.
The Dose Response Effect of Mother’s Own Milk
Breast milk is a powerful thing. Previous studies on the impact of mother’s own milk (MOM) have shown that with every increment of 10 mL/kg/d of average intake, the risk of such outcomes as BPD and adverse developmental outcomes are decreased. In the case of BPD the effect is considerable with a 9.5% reduction in the odds of BPD for every 10% increase in MOM dose. With respect to developmental outcome ach 10 mL/kg/day increase in MOM was associated with a 0.35 increase in cognitive index score.
One of the best names for a study has to be the LOVE MOM study which enrolled 430 VLBW infants from 2008-2012. The results of this study Impact of early human milk on sepsis and health-care costs in very low birth weight infants.indicated that with incremental increases of 10 mL/kg of MOM reductions in sepsis of 19% were achieved and in addition overall costs were reduced.
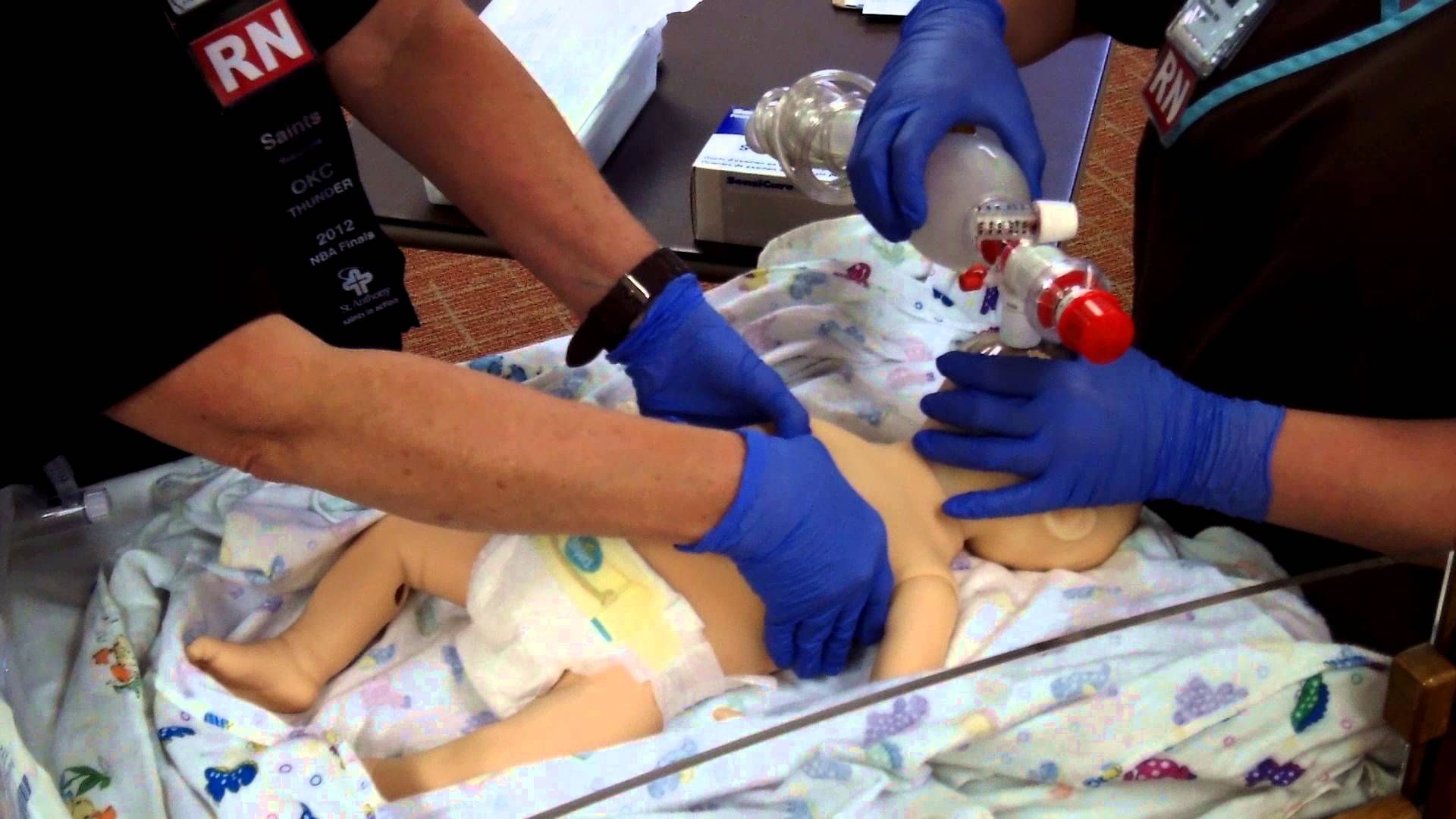
The same group just published another paper on this cohort looking at a different angle. NICU human milk dose and health care use after NICU discharge in very low birth weight infants. This study is as described and again looked at the impact of every 10 mL/kg increase in MOM at two time points; the first 14 and the first 28 days of life. Although the data for the LOVE MOM trial was collected prospectively it is important to recognize how the data for this study was procured. At the first visit after NICU discharge the caregiver was asked about hospitalizations, ED visits and specialized therapies and specialist appointments. These were all tracked at 4 and 8 months of corrected age were added to yield health care utilization in the first year, and the number of visits or provider types at 4, 8, and 20 months of corrected age provided health care utilization through 2 years.
What were the results?
“Each 10 mL/kg/day increase in HM in the first 14 days of life was associated with 0.26 fewer hospitalizations (p =
0.04) at 1 year and 0.21 fewer pediatric subspecialist types (p = 0.04) and 0.20 fewer specialized therapy types (p = 0.04) at 2 years.” The results at 28 days were not statistically significant. The authors reported both unadjusted and adjusted results controlling for many factors such as gestational age, completion of appointments and maternal education to name a few which may have influenced the results. The message therefore is that the more of MOM a VLBW is provided in the first 14 days of life, the better off they are in the first two years of life with respect to health care utilization.

That even makes some sense to me. The highest acuity typically for such infants is the first couple of weeks when they are dealing with RDS, PDA, higher oxygen requirements etc. Could the protective effects of MOM have the greatest bang for your buck during this time. By the time you reach 28 days is the effect less pronounced as you have selected out a different group of infants at that time point?
What is the weakness here though? The biggest risk I see in a study like this is recall bias. Many VLBW infants who leave the NICU have multiple issues requiring many different care providers and services. Some families might keep rigorous records of all appointments in a book while others might document some and not others. The big risk here in this study is that it is possible that some parents overstated the utilization rates and others under-reported. Not intentionally but if you have had 20 appointments in the first eight months could the number really by 18 or 22?
Another possibility is that infants receiving higher doses of MOM were healthier at the outset. Maternal stress may decrease milk production so might mothers who had healthier infants have been able to produce more milk? Are healthier infants in the first 14 days of life less likely to require more health care needs in the long term?
How do we use this information?
In spite of the caveats that I mentioned above there are multiple papers now showing the same thing. With each increment of 10 mL/kg of MOM benefits will be seen. It is not a binary effect meaning breastfed vs not. Rather much like the medications we use to treat a myriad of conditions there appears to be a dose response. It is not enough to ask the question “Are you intending to breastfeed?”. Rather it is incumbent on all of us to ask the follow-up question when a mother says yes; “How can we help you increase your production?” if that is what the family wants>
by All Things Neonatal | Sep 20, 2018 | resuscitation
One of the first things a student of any discipline caring for newborns is how to calculate the apgar score at birth. Over 60 years ago Virginia Apgar created this score as a means of giving care providers a consistent snapshot of what an infant was like in the first minute then fifth and if needed 10, 15 and so on if resuscitation was ongoing. For sure it has served a useful purpose as an apgar score of 0 and 0 gives one cause for real worry. What about a baby with an apgar of 3 and 7 or 4 and 8? There are certainly infants who have done very well who initially had low apgar scores and conversely those who had higher apgar scores who have had very significant deleterious outcomes including death. I don’t mean to suggest that the apgar scores don’t provide any useful predictive value as they are used as part of the criteria to determine if a baby merits whole body cooling or not. The question is though after 60+ years, has another score been created to provide similar information but enhance the predictive value derived from a score?
The Neonatal Resuscitation and Adaptation Score (NRAS)
Back in 2015 Jurdi et al published Evaluation of a Comprehensive Delivery Room Neonatal Resuscitation and Adaptation Score (NRAS) Compared to the Apgar Score. This new score added into a ten point score resuscitative actions taken at the 1 and 5 minute time points to create a more functional score that included interventions. The other thing this new score addressed was more recent data that indicated a blue baby at birth is normal (which is why we have eliminated asking the question “is the baby pink?” in NRP. Knowing that, the colour of the baby in the apgar score may not really be that relevant. Take for example a baby with an apgar score of 3 at one minute who could have a HR over 100 and be limp, blue and with shallow breathing. Such a baby might get a few positive pressure breaths and then within 10 seconds be breathing quite well and crying. Conversely, they might be getting ongoing PPV for several minutes and need oxygen. Were they also getting chest compressions? If I only told you the apgar score you wouldn’t have much to go on. Now look at the NRAS and compare the information gathered using two cardiovascular (C1&2), one neurological test (N1) and two respiratory assessments (R1&2).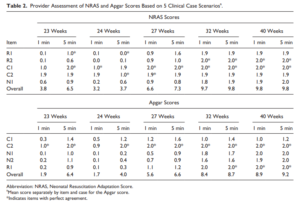

The authors in this study performed a pilot study on only on 17 patients really as a proof of concept that the score could be taught and implemented. Providers reported both scores and found “superior interrater reliability (P < .001) and respiratory component reliability (P < .001) for all gestational ages compared to the Apgar score.”
A Bigger Study Was Needed
The same group in 2018 this time led by Witcher published Neonatal Resuscitation and Adaptation Score vs Apgar: newborn assessment and predictive ability. The primary outcome was the ability of a low score to predict mortality with a study design that was a non-inferiority trial. All attended deliveries were meant to have both scores done but due to limited numbers of trained personnel who could appropriately administer both scores just under 90% of the total deliveries were assigned scores for comparison. The authors sought to recruit 450 infants to show that a low NRAS score (0–3) would not be inferior to a similar Apgar at predicting death. Interestingly an interim analysis found the NRAS to be superior to Apgar when 75.5% of the 450 were enrolled, so the study was stopped. What led the apgar score to perform poorly in predicting mortality (there were only 12 deaths though in the cohort) was the fact that 49 patients with a 1 minute apgar score of 0-3 survived compared to only 7 infants with a low NRAS score.
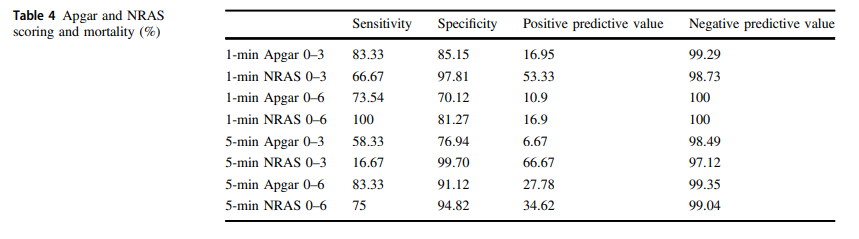
The other interesting finding was the ability of the NRAS to predict the need for respiratory support at 48 hours with a one minute apgar score of 0-3 being found in 39% of those on support compared to 100% of those with a low NRAS. Also at 5 minutes a score of 4-6 for the apgar was found in 48% of those with respiratory support at 48 hours vs 87% of those with a similar range NRAS. These findings were statistically significant while a host of other conditions such as sepsis, hypoglycemia, hypothermia and others were no different in terms of predictive ability of the scores.
An Even Bigger Study is Needed
To be sure, this study is still small and missed just over 90% of all deliveries so it is possible there is some bias that is not being detected here. I do think there is something here though which a bigger study that has an army of people equipped to provide the scoring will add to this ongoing story. Every practitioner who resuscitates an infant is asked at some point in those first minutes to hour “will my baby be ok?”. The truth is that the apgar score has never lived up to the hope that it would help us provide an accurate clairvoyant picture of what lies ahead for an infant. Where this score gives me hope is that a score which would at the very least help me predict whether an infant would likely still be needing respiratory support in 48 hours provides the basic answer to the most common question we get in the unit once admitted; “when can I take my baby home”. Using this score I could respond with some greater confidence in saying “I think your infant will be on support for at least 48 hours”. The bigger question though which thankfully we don’t have to address too often for the sickest babies at birth is “will my baby survive?”. If a larger study demonstrates this score to provide a greater degree of accuracy then the “Tipping Point” might just be that to switching over to the NRAS and leaving the apgar score behind. That will never happen overnight but medicine is always evolving and with time you the reader may find yourself becoming very familiar with this score!

by All Things Neonatal | Sep 6, 2018 | Neonatal, Neonatology, newborn, preemie
It has been a few months now that I have been serving as Chair of the Fetus and Newborn Committee for the Canadian Pediatric Society. Certain statements that we release resonate strongly with me and the one just released this week is certainly one of them. Guidelines for vitamin K prophylaxis in newborns is an important statement about a condition that thankfully so few people ever experience. To read the statement on the CPS website click here.
Similar story to vaccinations
Prior to the American Academy of Pediatrics in 1961 proclaiming that all newborns should receive IM Vitamin K at birth the incidence of Vitamin K deficient bleeding was 0.25 – 1.7%. Think about that for a moment. A new parent could expect that 1/100 babies roughly might have intestinal bleeding or worse an intracranial hemorrhage due to an insufficient amount of vitamin K levels in the newborn. The types of bleeding could be categorized into three different time epochs. Early onset (occurring in the first 24 hours post-birth), classic (occurring at days 2 to 7) and late onset (at 2 to 12 weeks and up to 6 months of age).
With a rate that high detractors of providing Vitamin K at birth would say “why should we give it; I haven’t heard of any baby getting such bleeding?” Looking at it another way though, why don’t you see congenital rubella or kids with measles much these days? It’s due to vaccination. Thankfully as a Neonatologist, I don’t see Vitamin K deficient bleeding since most parents provide Vitamin K to their babies at birth. If you went back to the era prior to 1961 when widespread supplementation of Vitamin K began in the US, I imagine it would not have been too uncommon to hear about a baby who had bleeding issues after birth. Just because we don’t hear about German Measles much anymore doesn’t mean the virus causing it doesn’t still exist!
How Effective is Vitamin K?
How effective is Vitamin K administration at birth in preventing hemorrhagic disease of the newborn (HDNB)? Studies estimate an incidence of 0.25 per 100000 live births or 1 in 400000 babies vs the 1/100 risk without any vitamin K. That is one effective intervention! At this point I would ask those families that are still concerned about giving Vitamin K to their infants if this is a risk they can accept? If they refuse Vitamin K and there is a significant bleed how will they react?
The Change in this CPS Statement From the Past
In the last statement on Vitamin K, the authors suggested that the oral route was a reasonable option. Instead of giving 1 mg of Vitamin K IM one would dose it as 2 mg orally and then repeat at 2-4 weeks and then 6-8 weeks. In looking at the effectiveness though it is worth noting that while we can assure that families will get the first dose, as with any medication that needs repeat dosing there is the risk of forgetfulness leading to missed dosing down the road. In fact when the authors looked at the risk of late HDNB they found the following “The relative risk for VKDB, when comparing PO versus IM vitamin K administration in these two studies, was 28.75 (95% CI 1.64 to 503.45) and 5.97 (95% CI 0.54 to 65.82), respectively [19][20].”
The outcome of course remains rare but the risk based on two studies was almost 30 times higher than if IM dosing was given.
On this basis IM is recommended.
Having said all this I recognize that despite all this information, some families will choose for a number of reasons to still opt for the oral dose. As the statement suggests we need to encourage such use when a family refuses IM vitamin K. The 30 fold risk compared to IM administration is magnitudes lower than the approximate 1/100 risk of giving nothing at all!
In the end I believe that one case of intracranial hemorrhage from inadequate vitamin K is too much. This one vitamin indeed could save a life.


 When looking at the rates of IVH and the severity of those affected the results are striking.
When looking at the rates of IVH and the severity of those affected the results are striking.


 It would have been nice to see mean results during this time rather than lowest but this is what we have.
It would have been nice to see mean results during this time rather than lowest but this is what we have.





