by All Things Neonatal | Jun 5, 2019 | resuscitation
Just about all of our preterm infants born at <29 weeks start life out the same in terms of neurological injury. There are of course some infants who may have suffered ischemic injury in utero or an IVH but most are born with their story yet to be told. I think intuitively we have known for some time that the way we resuscitate matters. Establishing an FRC by inflating the lungs of these infants after delivery is a must but as the saying goes the devil is in the details.
The Edmonton group led by Dr. Schmolzer has had several papers examined in these blogs and on this occasion I am reviewing an important paper that really is a follow-up study to a previous one looking at the impact of high tidal volume delivery after birth. I have written on this previous paper before in It’s possibile! Resuscitation with volume ventilation after delivery. On this occasion the authors have published the following paper; Impact of delivered tidal volume on the occurrence of intraventricular haemorrhage in preterm infants during positive pressure ventilation in the delivery room.This observational study had a simple enough premise. Will the use of Vt > 6 mL/kg in infants given PPV for at least two minutes lead to worse rates of IVH? All infants were < 29 weeks and if they had chest compressions or epinephrine were excluded. All infants were treated equally in terms of delayed cord clamping and antenatal steroid provision. Ventilation was done with a t-piece resuscitator and Vt measured with an NM3 monitor connected to the face mask. First ultrasounds were done for all at 3 days of age.
What did the authors find?
One hundred and sixty five infants comprised this cohort. Overall, 124 (75%) infants were in the high volume group compared to 41 (25%) with a mean VT<6 mL/kg. Median Vt were 5.3 (4.6-5.7) ml/kg for the low group and 8.7
(7.3-10.6) mL/kg which were significantly different.
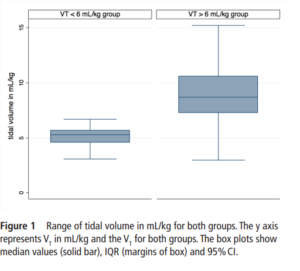
 When looking at the rates of IVH and the severity of those affected the results are striking.
When looking at the rates of IVH and the severity of those affected the results are striking.
IVH in the high VT group was diagnosed in 63 (51%) infants compared with 5 (13%) infants in the normal VT group (P=0.008).Severe IVH (grade III or IV) developed in 33/124 (27%) infants in the high VT group and 2/41 (6%) in the normal VT group (P=0.01)
Hydrocephalus, following IVH developed in 7/49 (14%) and 2/16 (13%) in the >6 mL/kg and <6 mL/kg VT groups. Looking at other factors that could affect the outcome of interest the authors noted the following physiologic findings. Oxygen saturations were lower in the low volume group at 6, 13 and 14 min after birth while tissue oxygenation as measured by NIRS was similarly lower at 7,8 and 25 min after birth (P<0.001). Conversely, heart rate was significantly lower in the VT>6 mL/kg group at 5, 20 and 25 min after birth (P<0.001). Fraction of inspired oxygen was similar in both groups within the first 30 min. Systolic, diastolic and mean blood pressure was similar between the groups. What these results say to me is that despite having lower oxygen saturations and cerebral oxygen saturation at various time points in the first 25 minutes of life the infants seem to be better off given that HR was lower in those given higher volumes despite similar FiO2. Rates of volume support after admission were slightly higher in the high volume group but inotrope usage appears to be not significantly different. Prophylactic indomethacin was used equally in the two cohorts.
Thoughts for the future
Once a preterm infant is admitted to the NICU we start volume targeted ventilation from the start. In the delivery room we may think that we do the same by putting such infants on a volume guarantee mode after intubation but the period prior to that is generally done with a bag and mask. Whether you use a t-piece resuscitator or an anesthesia bag or even a self inflating bag, you are using a pressure and hoping not to overdistend the alveoli. What I think this study demonstrates similar to the previous work by this group is that there is another way. If we are so concerned about volutrauma in the NICU then why should we feel any differently about the first few minutes of life. Impairment of venous return from the head is likely to account for a higher risk of IVH and while a larger study may be wished for, the results here are fairly dramatic. Turning the question around, one could ask if there is harm in using a volume targeted strategy in the delivery room? I think we would be hard pressed to say that keeping the volumes under 6 mL/kg is a bad idea. The challenge as I see it now is whether we rig up devices to accomplish this or do the large medical equipment providers develop an all in one system to accomplish this? I think the time has come to do so and will be first in line to try it out if there is a possibility to do a trial.

by All Things Neonatal | May 23, 2019 | resuscitation
We have all been there. After an uneventful pregnancy a mother presents to the labour floor in active labour. The families world is turned upside down and she goes on to deliver an infant at 27 weeks. If the infant is well and receives minimal resuscitation and is on CPAP we provide reassurance and have an optimistic tone. If however their infant is born apneic and bradycardic and goes on to receive chest compressions +/- epinephrine what do we tell them? This infant obviously is much sicker after delivery and when the family asks you “will my baby be ok?” what do you tell them? It is a human tendency to want to reassure and support but if they ask you what the chances are of a good outcome it has always been hard to estimate. What many of us would default to is making an assumption that the need for CPR at a time when the brain is so fragile may lead to bleeding or ischemia would lead to worse outcomes. You would mostly be right. One study by Finer et al entitled Intact survival in extremely low birth weight infants after delivery room resuscitation.demonstrated that survival for infants under 750g was better if they had a history of CPR after delivery. The thought here is that more aggressive resusctiation might be responsible for the better outcome by I would presume establishing adequate circulation sooner even if the neonates did not appear to need it immediately.
The Canadian Neonatal Network
In Canada we are fortunate to have a wonderful network called the Canadian Neonatal Network. So many questions have been answered by examining this rich database of NICUs across the county. Using this database the following paper was just published by Dr. A. Lodha and others; Extensive cardiopulmonary resuscitation of preterm neonates at birth and mortality and developmental outcomes. The paper asked a very specific and answerable question from the database. For infants born at <29 weeks gestational age who require extensive resuscitation (chest compressions, epinephrine or both) what is the likelihood of survival and/or neurodevelopmental impairment (NDI) at 18-24 months of age vs those that did not undergo such resuscitation? For NDI, the authors used a fairly standard definition as “any cerebral palsy (GMFCS�1), Bayley-III score <85 on one or more of the cognitive, motor or language composite scores, sensorineural or mixed hearing impairment or unilateral or bilateral visual impairment.” Their secondary outcomes were significant neurodevelopmental impairment (sNDI), mortality, a Bayley-III score of <85 on any one of the components (cognitive, language, motor), sensorineural or mixed hearing loss,or visual impairment.sNDI was defined as the presence of one or more of the following: cerebral palsy with GMFCS 3, Bayley-III cognitive, language or motor composite score <70, hearing impairment requiring hearing aids or cochlear implant, or bilateral visual impairment”
What did they discover?
It is a fortunate thing that the database is so large as when you are looking at something like this the number of infants requiring extensive resuscitation is expected to be small. The authors collected data from January 1, 2010 and September 30, 2011 and had a total number of infants born at less than 29 weeks of 2760. After excluding those with congenital anomalies and those who were born moribund they were left with 2587. From these 80% had follow-up data and when applying the final filter of extensive resuscitation they were left with 190 (9.2%) who received delivery room CPR (DR-CPR) vs 1545 who did not receive this.
Before delving into the actual outcomes it is important to note that neonates who did not receive DR-CPR were more likely to be born to mothers with hypertension and to have received antenatal steroids (89 vs 75%). With these caveats it is pretty clear that as opposed to the earlier study showing better outcomes after DR-CPR this was not the case here.

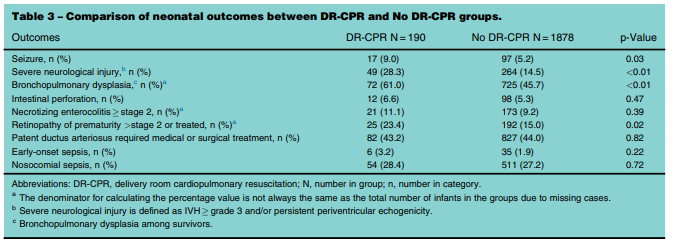
 The results are interesting in that it is pretty clear that receiving DR-CPR is not without consequence (higher rate of seizures, severe neurological injury, BPD). Looking at the longer term outcomes though is where things get a little more interesting. Mortality and mortality or neurodevelopmental impairment are statistically significant with respect to increased risk. When you take out NDI alone however the CI crosses one and is no longer significant. Neither is CP for that matter with the only statistically significant difference being the Bayley-III Motor composite score <85. The fact that only this one finding came out as significant at least to me raises the possibility that this could have been brought about by chance. It would seem that while these infants are at risk of some serious issues their brains in the long run may be benefiting for the neurological plasticity that we know these infants have.
The results are interesting in that it is pretty clear that receiving DR-CPR is not without consequence (higher rate of seizures, severe neurological injury, BPD). Looking at the longer term outcomes though is where things get a little more interesting. Mortality and mortality or neurodevelopmental impairment are statistically significant with respect to increased risk. When you take out NDI alone however the CI crosses one and is no longer significant. Neither is CP for that matter with the only statistically significant difference being the Bayley-III Motor composite score <85. The fact that only this one finding came out as significant at least to me raises the possibility that this could have been brought about by chance. It would seem that while these infants are at risk of some serious issues their brains in the long run may be benefiting for the neurological plasticity that we know these infants have.
The study is remarkable to me in that an infant can have such a difficult start to life yet hope may remain even after dealing with some of the trials and tribulations of the NICU. Parents may need to wade through the troubling times of seizures, long term ventilation and CPAP and then onto a diagosis of BPD but their brains may be ok after all. This is one of the reasons I love what I do!

by All Things Neonatal | May 1, 2019 | IVH
Recently the practice of keeping ELBW infants with a midline head position for the first three days of life has been recommended to reduce IVH as part of a bundle in many units. The evidence that this helps to reduce IVH has been somewhat circumstantial thus far. Studies finding that decreased sagittal sinus blood flow, increased cerebral blood volume with increased intracranial pressure all occur after head turns would theoretically increase the risk of IVH. Raising the head of the bed would help in theory with drainage of the venous blood from the head and in fact systemic oxygenation has been shown to improve with such positioning. This presumably is related to increased cardiac output from better systemic venous return.
Bringing it to the bedside
Interestingly, some of the above studies are from over thirty years ago. We now have some evidence to look at involving this practice. Kochan M et al published Elevated midline head positioning of extremely low birth weight infants: effects on cardiopulmonary function and the incidence of
periventricular-intraventricular. The study involved maintaining ELBW infants in an elevated midline head position (ELEV- supine, head of bed elevated 30 degrees, head kept in midline) versus standard head positioning (FLAT–flat supine, head turned 180 degrees every 4 h) during the first 4 days of life to see if this would decrease in the incidence of IVH. Ninety infants were randomized into both arms of the study. In terms of baseline characteristics, BW of 725g in the FLAT vs 739 in ELEV were comparable as well as GA both at 25 weeks. Two differences on the maternal side existed of 40% ELEV vs 24.4% FLAT of mothers having preeclampsia and 23.3% FLAT vs 10% ELEV having prolonged rupture of membranes both of which were statistically significant.
What did they find?
Ultrasounds were performed at entry into the study and then daily for days 1-4 and then on day 7 with abnormal scans repeated weekly. In terms of IVH the authors noted no overall difference in rate of IVH. What they did find however was a statistically significant reduction in the rate of Grade IV IVH.
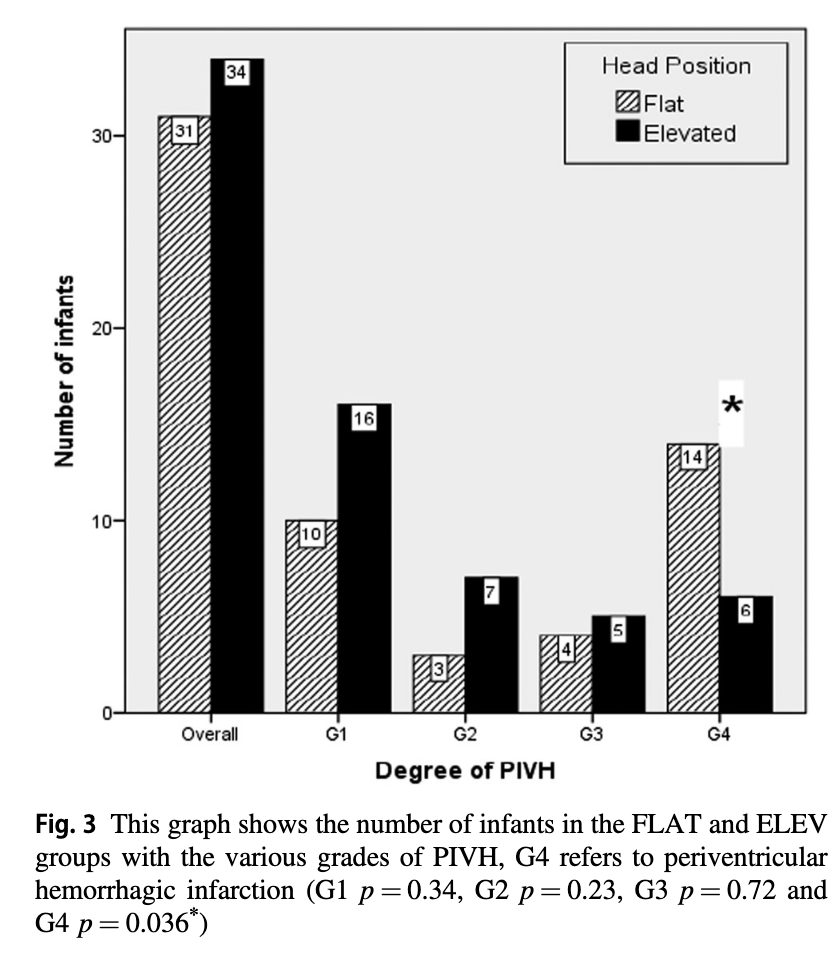
The p value for the finding of lower rates of Grade IV IVH was 0.036 so not strikingly significant but different nonetheless. Given that the venous drainage of the head is also dependent on the resistance to flow from the pressure in the thorax one can’t infer that the intervention alone is responsible for this without ensuring that that respiratory findings are similar as well. Similarly without knowing inflow of blood into the head as measured by blood pressure it is difficult to say that the reduction in IVH isn’t related to differences in blood pressure.
The authors helpfully looked at both of these things. For those infants on high frequency ventilation the mean airway pressure was higher on day one being 11.5 cm H2O (FLAT) vs 9.9 cm H2O (ELEV) neither of which are high although different. The rest of the three days were no different. For those on conventional ventilation the only difference was on day 4 where the MAP was higher for ELEV at 8 vs 7.4 cm H2O which again is fairly mild. Interestingly, as was found in other studies that oxygenation was improved with elevation of the head, the maximum FiO2 for the two groups was different on day 1 being 46% in the FLAT vs 37.5% in the ELEV.
Looking at the hemodynamic side of things there were differences in the lowest mean BP recorded on day 1 and 3 but otherwise the groups were similar.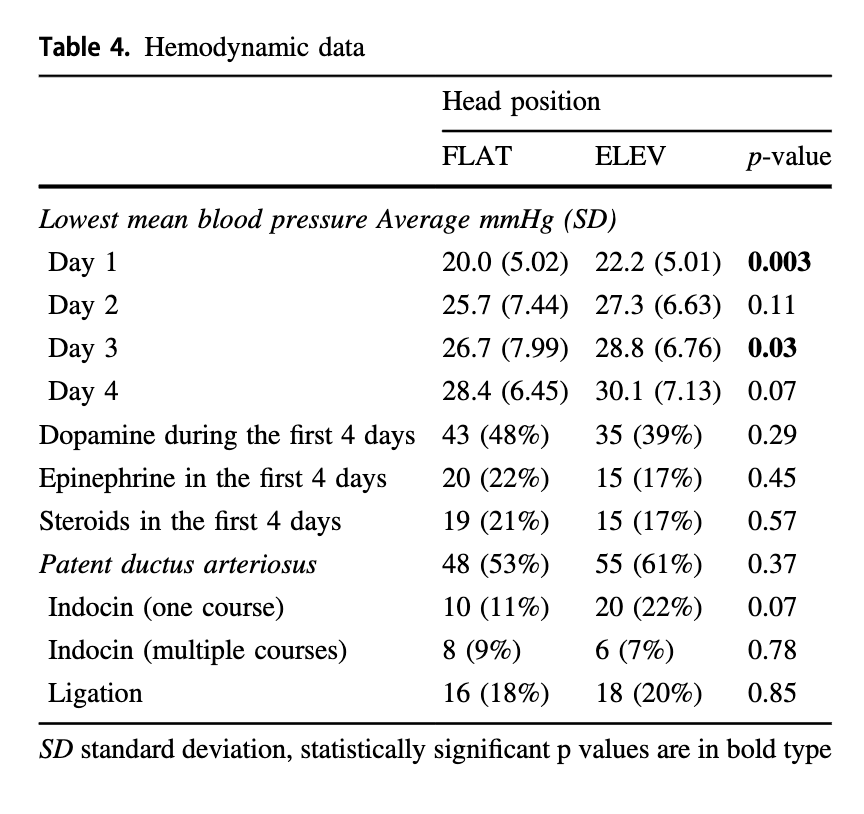 It would have been nice to see mean results during this time rather than lowest but this is what we have.
It would have been nice to see mean results during this time rather than lowest but this is what we have.
In terms of complications of preterm birth there were no differences found in rates of sepsis (important given the increase rate of prolonged rupture in the FLAT group), NEC or ROP.
Although length of stay was no different 92 vs 109 days ELEV (NS), survival to discharge was at 88% vs 76% (p=0.033) which also may explain the longer length of stay.
What Can We Learn From This
Don’t worry. I am not about to throw the results out. There are a couple observations though that need to be addressed. The first is the increased rate of preecampsia in the ELEV group. This finding could have impacted the results. We know that fetuses exposed to this condition are stressed and are often born with better lungs than their non-exposed counterparts. The endogenous increase in steroids due to this stress is attributable and may explain the better oxygenation and lower mean airway pressures needed in the ELEV group rather than improvements in flow alone from positioning. The second issue is adherence to the protocol as there were some infants in the ELEV group who were placed flat for the final 1-2 days of the study. Having said that, this would serve to dilute the effect rather than strengthen it so perhaps it makes the results more believable.
So where does this leave us? This study demonstrates improved survival and a reduction in Grade IV IVH without an overall reduction in IVH. There was nothing found to suggest that the intervention is harmful. Given the background studies demonstrating improved systemic oxygenation, reductions in ICP and cerebral blood volume the finding of reduced severe IVH seems plausible to me. This could be a practice changing study for some units who have perhaps only adopted midline positioning in the first few days of life. It will be interesting to see if this takes off but is certainly worth a good look at.

by All Things Neonatal | Apr 10, 2019 | nutrition
The medical term for this is placentophagy and it is a real thing. If you follow the lay press you may have seen that originally this was promoted by Kourtney Kardashian who did this herself and then by Kim who planned on doing the same after delivery. See Did Kourtney Kardashian Eat Her Placenta? 
This is not completely without basis as many readers will be thinking already that they have heard about the health benefits of doing the same. Reports of improved mood and reductions in the baby blues following ingestion of placenta as well as improvements in breast milk production have led to this growing practice. The evidence for this up until recently though was quite old and fraught with poorly design of such studies. The bigger driver however has been word of mouth as many women having heard about the promises of better mood at the very least have thought “why not? Can’t hurt.”
What I will do in this post is run through a little background and a few recent studies that have shed some light on how likely this is to actually work.
Where did the idea come from?
Animals eat their placentas after delivery. It turns out that unprocessed placenta is quite high in the hormone prolactin which is instrumental for breastfeeding. Given the large amount of this hormone as well as the number of other hormones present in such tissue it was thought that the same benefits would be found in humans. Eating unprocessed human tissue whether it is put in a capsule or not is unwise as unwanted bacteria can be consumed. In fact, a case of GBS sepsis has been linked to such a practice in which the source of the GBS was thought to be due to contaminated unprocessed maternal placenta that had been ingested. Buser GL, Mat´o S, Zhang AY, Metcalf BJ, Beall B, Thomas AR. Notes from the field: Late-onset infant group B streptococcus infection associated
with maternal consumption of capsules containing dehydrated placenta.
What happens when you process placenta by steaming and drying?
This would be the most common way of getting it into capsules. This process which renders it safe to consume may have significant effects on reducing hormonal levels.This was found in a recent study that measured oxytocin and human placental lactogen (both involved positively in lactation) and found reductions in both of 99.5% and 89.2%, respectively compared versus raw placenta. I would assume that other hormones would be similarly affected so how much prolactin might actually wind up in these capsules after all?
Clinical Randomized Double Blind Controlled Trial
Twenty seven women from Las Vegas were recruited into a pilot trial (12 beef placebo vs 15 steamed and dried placenta) with the authors examining three different outcomes across three studies. The first study Effects of placentophagy on maternal salivary hormones: A pilot trial, part 1 looked at a large number of salivary hormones at four time points. Plasma samples were taken as well to determine the volume of distribution of the same. First samples were at week 36 of gestation then within 4 days (96 h) of birth followed by days 5–7 (120–168 h) postpartum and finally Days 21–27 (504–648 h) postpartum. All consumption of capsules was done in the home as was collection of samples. As per the authors in terms of consumption it was as follows “two 550 mg capsules three times daily for the first 4 days; two 550 mg capsules twice daily on days 5 through 12, and then to decrease the dose to two 550 mg capsules once daily for the remainder of the study (days 13 through approximately day 20 of supplementation).
Outcomes
No difference was found between salivary concentrations of hormones at any time point other than that with time they declined following birth. Curiously the volume of distribution of the hormones in serum was slightly higher in the placenta capsule groups but not enough to influence the salivary concentrations. It was felt moreover that the amount of incremental hormone level found in the serum was unlikely to lead to any clinical response.
The second study was on mood Placentophagy’s effects on mood, bonding, and fatigue: A pilot trial, part 2. Overall there were no differences for the groups but they did find “some evidence of a decrease in depressive symptoms within the placenta group but not the placebo group, and reduced fatigue in placenta group participants at the end of the study compared to the placebo group.”
The last paper published from the same cohort is Ingestion of Steamed and Dehydrated Placenta Capsules Does Not Affect Postpartum Plasma Prolactin Levels or Neonatal Weight Gain: Results from a Randomized, Double-Bind, Placebo-Controlled Pilot Study. This study specifically addressed the issue of prolactin levels and found no difference between the groups. Neonatal weight gain was used as a proxy for breastmilk production as it was thought that if there was an effect on breastmilk you would see better weight gain. About 80% in both groups exclusively breastfed so the influence of formula one can’t take out of the equation. In the end weight gain was no different between groups although a trend to better weight gain was seen in the placebo group.
To eat or not to eat that is the question?
What is clear to me is that the answer to this question remains unclear! What is clear is that I don’t think it is wise to consume raw placenta due to the risks of bacterial contamination. Secondly, the levels of hormones left in the placental preparation and the most common preparation of steaming and drying leave hormone levels that are unlikely to influence much at all from a biochemical standpoint. It also seems that breastmilk production and neonatal weight gain aren’t influenced much by consumption of these pills.
The issue though in all of this is that while the previous research was of low quality, the current research while of better quality is at a low volume. These were pilot trials and not powered to find a difference likely. The finding in the subgroup of some effect on mood at the end of the study does leave some hope to those that believe in the power of the placenta to help. Would a larger study find benefit to this practice? My suspicion from a biochemical standpoint is not but that one may feel a benefit from a placebo response.
Should you go out and have your placenta prepared for consumption? If you have Kardashian like wealth then go for it if you think it will help. If you don’t then I would suggest waiting for something more definitive before spending your money on placentophagy.

by All Things Neonatal | Mar 21, 2019 | steroids
I feel like this has been a story in the making for some time. Next to caffeine, the story of prophylactic hydrocortisone must be one of my more popular topics and has been covered more than once before as in A Shocking Change in Position. Postnatal steroids for ALL microprems or Early Hydrocortisone: Short term gain without long term pain. and the last post Hydrocortisone after birth may benefit the smallest preemies the most! After reporting on this topic about once a year, a recent paper may wrap it all up in a bow for the holidays and present to us the conclusion after all this work on the topic. I was extremely interested in this topic not just because I believe this therapy may have a future in the standard approach to neonatal care for VLBWs but because I have served on the CPS Fetus and Newborn committee with two of the authors of the paper. Dr. Lacaze and Dr. Watterberg have an exceptional understanding of this topic and so when they band together with other experts in the field I take notice.
An Individual Patient Data Meta-Analysis
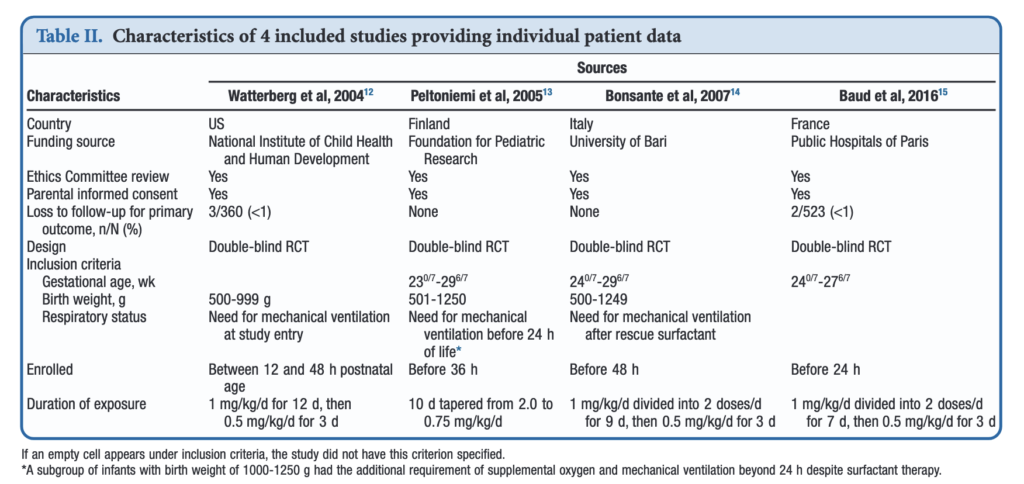
If you have read my previous posts then you know the story of why hydrocortisone given over the first 10-12 days of life might help those born before 30 weeks or < 1250g. In essence the concept is that it has been shown previously that many infants with relative adrenal insufficiency may go on to develop BPD. If you treat all such infants at risk you could theoretically reduce BPD. Typically after a few studies examining a similar topic come out, one can combine them in a meta-analysis using aggregate data (averages of effect sizes for the individual studies) and see what the larger sample shows. Another way to do it though is to go back to the original data and examine the infants at a more granular level allowing a greater identification and control of variables that might influence outcomes. This is what the authors led my Michele Shaffer did here in the paper Effect of Prophylaxis for Early Adrenal Insufficiency Using Low-Dose Hydrocortisone in Very Preterm Infants: An Individual Patient Data Meta-Analysis. There were a total of 5 studies on this topic but one study of 40 patients no longer had individual data so was excluded from analysis leaving 4 to look at. The details of the four studies are shown below. You can see that the inclusion criteria differed slightly but in general these were all infants up to 27 – 29 completed weeks and 500 – 1250g maximum who were treated with regimens as shown in the table.
What were the results?
Treatment with early low-dose hydrocortisone was associated with greater odds of survival without BPD at 36 weeks PMA after adjustment for sex, gestational age, and antenatal steroid use (aOR, 1.45; 95% CI, 1.11-1.90; I 2 = 0%). Also found were lower individual odds of BPD (aOR, 0.73; 95% CI, 0.54-0.98; I 2 = 0%), but not with a significant decrease in death before 36 weeks PMA (aOR, 0.76; 95% CI, 0.54-1.07; I 2 = 0%). Importantly although death by 36 weeks was not different, a decrease in death before discharge (aOR, 0.70; 95% CI, 0.51-0.97; I 2 = 0%) was found. Also noted and important was a reduction in medical treatment for PDA OR 0.72 (0.56-0.93)
All of these outcomes sound important but in a subgroup analysis other interesting findings emerged.
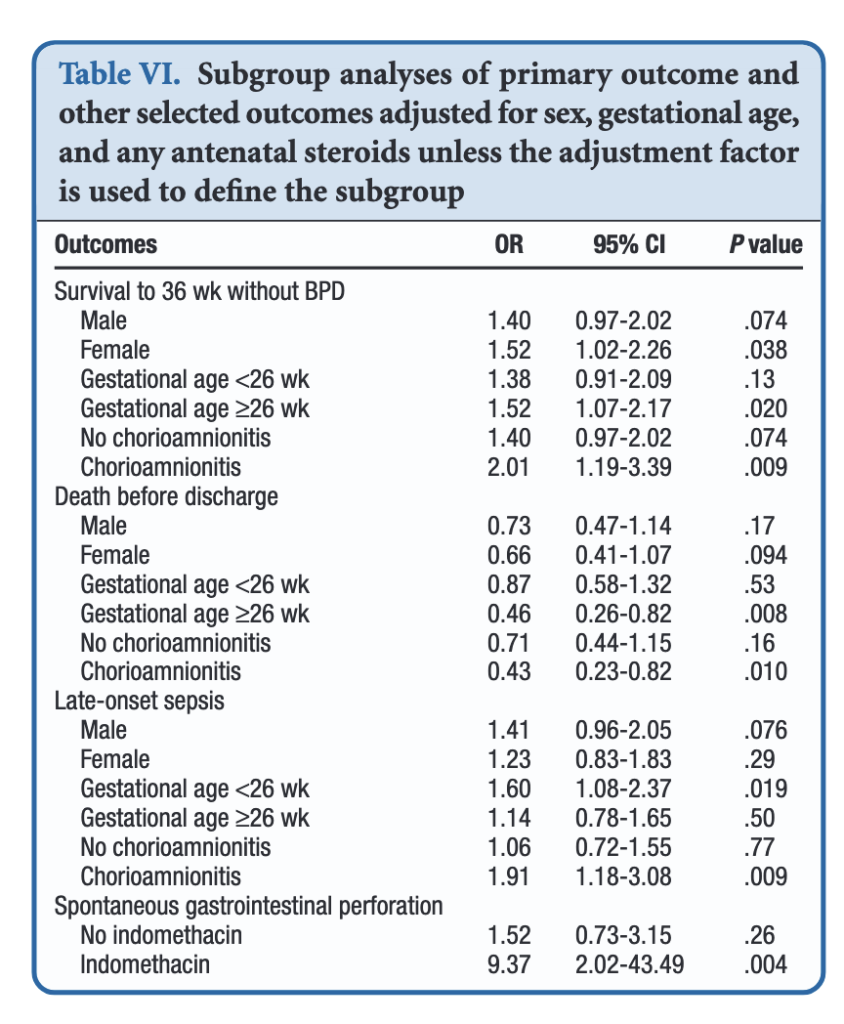
When dividing the patients into those less then 26 weeks and those at or greater than that gestational age, the benefits appear to be limited to those in the latter group. Levels of significance are high once you reach that GA suggesting that issues affecting those at younger gestational ages are less amenable to treatment. On the other hand one could say that the benefits seen at 26 – 29 weeks GA are relatively strong using a glass is half full approach. An important outcome worth noting is that while spontaneous intestinal perforation is noted to be a risk with prophylactic hydrocortisone, when you remove indomethacin from the equation the risk disappears. For those units using prophylactic hydrocortisone one would likely need to choose between the two but if you are like our unit where we don’t have that option this may be one strategy to consider.
In terms of risk to giving such therapy the big one noted in the paper was an increase in risk for late onset sepsis. Interestingly, this was limited though to the group under 26 weeks GA. In essence then the messaging would appear to be that under 26 weeks there may be less benefit to such treatment and therefore the increased risk of late onset sepsis without such benefits on BPD would suggest not using it in this GA group.
Where do we land then?
It would be easy to cast this aside I suppose as the group you are most worried about (22-25 weeks) doesn’t seem to really benefit but has a risk of late onset sepsis. That leaves us though with the group from 26-29 weeks. They do seem to benefit and may do so to a significant degree. They do develop BPD and to be honest we don’t have much outside of trying our best to use gentle ventilation to ameliorate their course in hospital. It is worth noting that the one group that does seem to show the greatest benefit are those exposed to chorioamnionitis. It is this group in particular that may be the best target for this intervention and I gather this has been discussed at a recent EPIQ meeting.
If one says no to trying this approach then the question that needs to be asked is whether doing nothing for this group is better than supporting them with hydrocortisone? If your centre’s rates of BPD are top notch then maybe you don’t want to add something in. If not though maybe it is time to rock the boat and try something different.


 When looking at the rates of IVH and the severity of those affected the results are striking.
When looking at the rates of IVH and the severity of those affected the results are striking.






 It would have been nice to see mean results during this time rather than lowest but this is what we have.
It would have been nice to see mean results during this time rather than lowest but this is what we have.



