
by All Things Neonatal | Sep 16, 2016 | Uncategorized
Campaign Closed October 13,2016! Thank you everyone for the $9359.00 raised!
First off I would like to thank everyone who has contributed to the fundraiser that All Things Neonatal has organized. Each gift no matter how small makes a difference one person at a time. I am overwhelmed not just by the generosity of the people who follow the Facebook page and this blog but by the numbers of people that have an interest in well… All things that are neonatal! The same day as the campaign began the Facebook page passed 10000 likes and that is saying something. When I started I had no idea where this would go and here we are just a year and a half in and by definition we can now say goodbye to our classification as a town and consider ourselves a city having reached 10000!
With respect to the subject of generosity though I would like to make special mention and a public thank you to two very large donations that came today. The first is from Vickar Community Chevrolet who gave $500 towards the cause. Given that I was looking for donations as small as $1 per member on the site I consider this to be quite amazing. It is quite inspiring to think that a company operating completely outside of the health care field still takes the time to recognize the importance of the needs of babies in our hospital.
The second “shout out” I feel the need to give is to a company that to be honest I had not heard of until today. The company is The Nanny Company Inc who operate out of Winnipeg and I am sure would be of use to many of the families that I have discharged who themselves may find themselves in need of such help. The Nanny Company today gave $1000 towards the fundraiser and with their donation I am proud to say we crossed the first threshold of $2000 and I am proud to announce we can officially buy one milk warmer for the NICU in Winnipeg!
By the way the Nanny Company also have a Facebook page as do Vickar Community Chevrolet so check them out to see what they do!
It is these kind of selfless acts that make me believe in the power of community. Whether you live in Winnipeg or not it doesn’t really matter. We all share space on this planet of ours and whether a baby is in Winnipeg, LA, Auckland or Capetown they all need our help. With that I ask you again whether you have followed this page or not if you can spare the cost of a cheap cup of coffee and use the power that we have with 10000 members to reach our goal of at least 4 milk warmers. If you would consider it click on this link below to Hold Their Hand and do something that you will feel great about this Weekend!
Hold Their Hand

by All Things Neonatal | Sep 15, 2016 | Neonatal, Neonatology, Uncategorized
Campaign Closed October 13,2016! Thank you everyone for the $9359.00 raised!
Each day the number of people following these sites grows and at the time of this post, the largest following on Facebook has over 8200 people who receive the feed on a daily basis. That is nothing short of remarkable and I hope that each of you gets something out of my writings and postings. I recognise that each post may not “light it up” in your mind but if you get at least a few “a ha” moments along the way then I am very happy that you have found these sites!
What This Is Not!
As I begin hinting at money, many of you may be thinking “here we go”, he is finally asking for some payment for this site! To be clear I have no interest in personal financial gain from this hobby I have developed, but rather find my joy in sharing ideas, getting your feedback and helping to generate interest overall in topics pertaining to Neonatology. I have no intention of ever asking for such payment but that doesn’t mean that I might not want to help someone else. For those of you who make philanthropy a part of your lives you will know the joy that comes from helping others. Being able to help others need not take tremendous dollars per donor when you have many people banding together to help a cause. This is the power that I am hoping to harness through this initiative and make a difference in care to our babies in hospital.
For the past year and a half, I have put my fingers to the keyboard to hopefully share my knowledge and expertise with you about an industry I am so passionate about.
My Philanthropic Side
When I am not busy finding content for the sites or being a Neonatologist, I am quite dedicated to philanthropy. One thing people may not realise about our province/country is that the government helps out the best they can financially but with the heavy demands of our province, they can’t meet all the needs. That’s why I’m proud of my partnership with the Children’s Hospital Foundation of Manitoba. The Foundation’s donors have helped bridge the gap so our hospital doesn’t go without the specialised items they need. From ultrasounds, starting a breast milk depot, specialised pediatric equipment and funding a position to support Quality Improvement in our unit to a soon to be announced Family Support coordinator position and so much more. But now, I turn to you to help us make the next difference in our unit.
The other day as the Facebook page hit 8,000 followers a thought struck me. What if I asked everyone on the page to just give $1 towards the purchase of a piece of equipment for babies in our units?
Hold Their Hand
In the Neonatal Intensive Care Unit (NICU), they are watched closely to make sure they are getting the right balance of fluids and nutrition. Incubators or special warmers help babies maintain their body temperature. This reduces the energy the babies have to use to stay warm and allow them to use that energy elsewhere.
Premature babies need to receive good nutrition so they grow at a rate close to that of babies still inside the womb. Babies born under 38 weeks have different nutritional needs than babies born at full term (after 38 weeks). They often have problems feeding from a bottle or a breast. This is because they are not yet mature enough to coordinate sucking, breathing, and swallowing.
Many NICUs will give donor milk from a milk bank to high-risk babies who cannot get enough milk from their own mother. But because the baby must be kept at a certain temperature to stay warm, so does their milk. 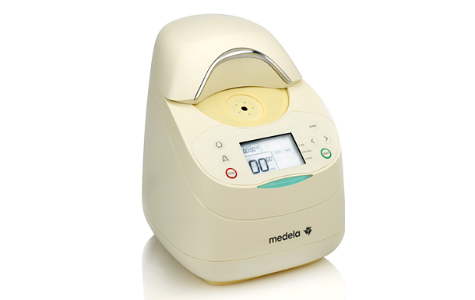
Thanks to the generous support of donors to the Children’s Hospital Foundation of Manitoba, 12 milk warmers have been purchased. However, we need 24 more warmers to keep up with demand. Each one costs $2,000 and will make a huge impact. An impact to help our babies get the nutrition they need at the temperature they require to survive and thrive.
So let’s hold their hand and let’s do it together! Has this journey of learning been worth at least $1 to you? If it has, then please help make a difference by giving at least $1. Giving more will only increase the power of this campaign! If you aren’t able to donate $1 or more, I ask that you share this post and challenge your friends to help make a difference to the over 1,000 patients we see a year. Click the link below to donate and make your difference today.
chfm.convio.net/help-hold-their-hand

by All Things Neonatal | Sep 7, 2016 | apnea of prematurity, Uncategorized
A question that we are asked from time to time is whether a home apnea monitor should be purchased after discharge from the hospital. The typical parent is one who has experienced the ups and downs of apnea of prematurity and is faced with the disturbing notion of coming off monitors and going home. “What if he has an event at home and I don’t know”? This leads to a search on the web for home monitors which finds numerous options to choose from. This is where things get interesting from a North American perspective.
In the two centres I have worked at in Canada our answer to such a question is to save your money and not buy one. Contrast this with two families I know in the US who were sent home by the hospital with home apnea monitors. How can the advice between the two nations be so different? I suspect the great risk of a lawsuit in the US is responsible at least in part but it may have to do with risk tolerance as well.
What does the evidence say?
First off, one might surmise that the use of a home apnea monitor helps hospitals move patients to the home faster than those centres that don’t prescribe them. A 2001 Cochrane systematic review on the subject noted that this was not the case and determined that out of nearly 15000 neonates studied the greatest predictor of sending such babies home on monitors was physician preference.
In the largest home monitoring study of its kind, the Collaborative Home Infant Monitoring Evaluation (CHIME) demonstrated some very important information. First off, ex-preterm infants have events and some of them quite significant after discharge. What the study which followed discharged infants at risk of SIDS in the home environment found though was that term infants also have events although less severe. Does this mean that everyone should run out and buy such monitoring equipment though? No! The main reason was that while the study did show that events may continue after discharge, it failed to show that these events had any relation to SIDS. The apneic events noted in hospital disappeared long before the arrival of a risk for SIDS. They really are separate entities.
The other issue with such monitors pertains to false alarms which can lead to sleepless nights, anxiety in parents and eventual abandonment of such technology. This led the AAP in 2005 to declare that they did not endorse such practice. Having said that, it is clear from my own experience with two US ex-preterm infants that this practice remains alive and well.
Could this be the solution?
One of my followers sent me this tonight and I have to say at the very least I am intrigued. The device is called the Owlet and was featured in this article The Sock That Could Save Your Babies Life. 
Watch the video here.
This monitor has me a little excited as it brings the home apnea monitor into the modern era with smart phone connectivity and at the same time helps the developers of this technology use data collected every two seconds to get a clearer picture on breathing patterns in infants that have been sent home. The saturation monitor in a sock is at the core of this technology which is meant to keep the probe in a relatively stable location. It brings another angle to the concept of wearable tech! What I find most interesting is the claim by the manufacturer that the device has a false alarm rate similar to that of a hospital saturation probe which would make it quite reliable.
I note though that the product has not received FDA approval yet (at least on the source I looked at) but is being worked on. The challenge though is whether this will truly make a difference. It may well have an excellent detection rate and it may in fact detect true apnea leading to bradycardia and cyanosis. What it won’t do though is change the natural history of these events once home. It may capture them very well but I suspect the four events that the mother in the video describes may have been self resolving if she hadn’t intervened. We know from the CHIME study that the events seen in the home did not lead to death from SIDS so I see no reason why these would be different.
Is it useless?
I suppose that depends on your perspective. From a data collection point, obtaining data every two seconds in a cloud based storage environment will allow this company to describe the natural history of respiratory patterns in ex-preterm infants better than I suspect has ever been done before. From a population standpoint I suppose that is something! At an individual level I suppose it depends on your strength of “needing to know”. This may well be the best monitor out there and it may one day be the most reliable. Will it save your baby’s life? I doubt it but might it give you peace of mind if it false alarms very infrequently? I think it just might but based on the low likelihood of it changing the outcome of your baby you won’t see me recommending it. If I come across one make no mistake about it, I will want to play with it myself!
by All Things Neonatal | Aug 25, 2016 | Infection, Uncategorized
As I said in another post on this topic I have been a huge advocate of RSV prophylaxis since my days as a Pediatric resident. When I started my residency we were not using Palivizumab (Synagis) and I recall admitting 10+ patients per day at times with bronchiolitis. With the use of passive immunization this rate dropped dramatically in Manitoba although rates in other areas of the country may have not seen such significant impacts. Manitoba may be somewhat different from many areas due to the communities in Nunavut being so impacted when RSV enters these areas and can infect many of the children due to crowded living conditions and inability to really isolate kids from one and other. The lack of benefit in other areas though, has no doubt led to controversy among practitioners who often wonder if giving 5 IM injections during the RSV season is indeed worth it. The real question has not necessarily been does it work but to whom should it be given so that you get the most benefit.
A Big Change in The Last Year
In 2015 the CPS published a revised statement entitled Preventing hospitalizations for respiratory syncytial virus infection. This statement included a significant change to the recommendation for those who should receive the product.
- In preterm infants without CLD born before 30 + 0 weeks’ GA who are <6 months of age at the start of RSV season, it is reasonable (but not essential) to offer palivizumab. Infants born after 30 + 0 weeks’ GA have RSV admission rates that are consistently ≤7% (Figure 3), yielding a minimum number needed to treat of 18 (90 doses of palivizumab to prevent one RSV admission) if one assumes 80% efficacy and five doses per infant. Therefore, palivizumab should not be prescribed for this group.
Gone are recommendations for treating those from 30 – 32 weeks and moreover 33- 35 weeks if meeting certain conditions. There is a provision for those in Northern communities to expand these criteria to 36 0/7 weeks if such infants would require medical transport to receive care for bronchiolitis. What is not really clear though is what is meant by Northern communities in terms of criteria to determine suitability exactly.
Incidentally, the criteria are not so different than the AAP statement from August 2014. In their version of the statement they state:
“The burden of RSV disease and costs associated with transport from remote locations may result in a broader use of palivizumab for RSV prevention in Alaska Native populations and possibly in selected other American Indian populations.”
The American guideline also states that it is for those infants who are “well” and under 29 weeks that RSV prophylaxis is appropriate but from 29 – 32 weeks use should be restricted to those babies who are on oxygen at 28 days of life.
AAP News Release From This Week
As stated above there are those who have always been sceptical of the true cost benefit of RSV prophylaxis and I would imagine those individuals must have latched on to the following report. Otherwise healthy premature infants 29 weeks gestation and over unlikely to benefit from palivizumab
The authors of this study found that while during the RSV season admission for RSV bronchiolitis was lower from 29 – 32 weeks in those infants who had received Synagis. This would argue that it should be given in this group except for the fact that as the authors state it was a “wash” since hospitalization for non-RSV bronchiolitis in the same population was increased by a similar amount. In essence if you didn’t get RSV you wound up getting something else that still put you in hospital. The conclusion here is that the decision to drop the criterion for prophylaxis to under 29 weeks is supported since from 29 – 32 weeks you can’t prevent hospitalization from viruses that take the place of RSV.
A Few Thoughts Though Before We Conclude It Has No Place
As I stated upfront I am not totally free of bias having seen a very large impact up here in Manitoba. What I worry about though is that we have in medicine a tendency to try and capture the “gist” of a guideline rather than committing it in its entirety to memory. We can’t help ourselves as the volume of information we are asked to remember is growing daily. What this may lead to however is changes to our practice that may expose vulnerable infants. The AAP guideline was designed to recommend changes for the otherwise healthy infant under 29 weeks. What I think we are really talking about are the truly exceptional babies. In our institution babies born at less than 29 weeks and certainly those closer to 24 weeks often spend as much as a month on CPAP. At 28 days even if the patient were on room air it might only be due to the fact that they were on distending pressure. Put them on nasal prongs and they would qualify.
Another important consideration is the remote location point. 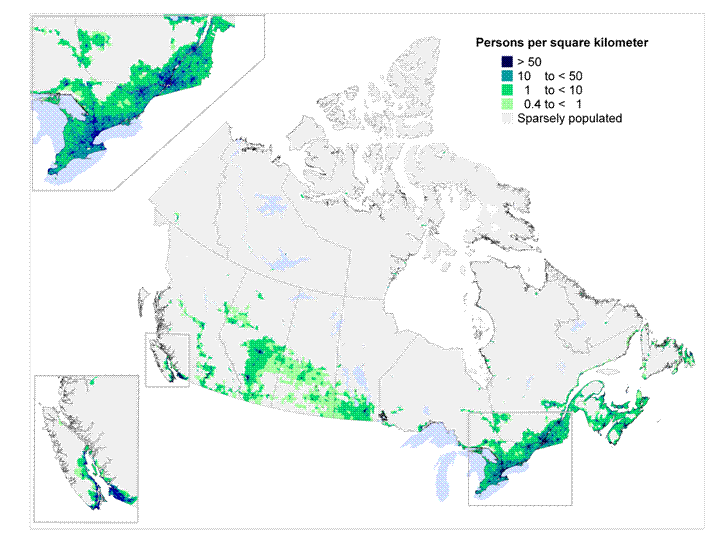 Here in Canada the majority of the population lives along the border with the US. Take a look though at our population density and you can see that for our Aboriginal (Indian) and Inuit populations as well as all those living in the north we have a significant need to protect them. Living conditions in these places involve overcrowding and high smoking levels both ideal breeding grounds for transmission of RSV and an intolerance to handling the inflammation in your bronchioles. The same is likely true of many parts of Alaska for my US readers.
Here in Canada the majority of the population lives along the border with the US. Take a look though at our population density and you can see that for our Aboriginal (Indian) and Inuit populations as well as all those living in the north we have a significant need to protect them. Living conditions in these places involve overcrowding and high smoking levels both ideal breeding grounds for transmission of RSV and an intolerance to handling the inflammation in your bronchioles. The same is likely true of many parts of Alaska for my US readers.
It would be too easy to simply state “I only need to give Synagis to those babies under 29 weeks now” but that would do a disservice to the populations in our remote communities both here and in the US.
In case you are wondering, I am not employed by the makers of Synagis here or in the US nor do I have any financial or conflicts otherwise. I am someone I suppose who has seen the difference of before and after and while I will let others debate the merits of giving Synagis from 29 – 32 weeks and above, I wrote this as a reminder that not all populations are the same and therefore we should not paint all susceptible patients with one brush.
by All Things Neonatal | Aug 18, 2016 | quality improvement, Uncategorized
The giant leaps in Neonatology may for the most part be over. So many outstanding research trials have brought us to where we are today. The major innovations of surfactant replacement, the discovery of nitric oxide and its later use to treat pulmonary hypertension, caffeine for apnea have all changed our field for the better. Cooling for HIE has certainly changed my practice in that I now truly have no idea what to tell parents after even some of the worst cases of asphyxia as our team has witnessed “miracles”after cooling. What will come next? My bet is that we are about to enter the era of Quality Improvement more and more. Think about the last study you read that had a major change in your practice or better yet made a substantial change in survival or neurodevelopmental outcome.
Tweaking care is where its at.
I like to think of it as fine tuning.  As the era of the major leaps in care seems to be passing us by what I see more and more are studies looking at how to make further improvements on what we already know. In some cases such as using higher doses of caffeine may reduce the incidence of apnea further compared to standard dosing while cooling for 96 hours instead of 72 and at lower temperatures after asphyxia may not be such a good idea after all. There will be some studies that suggest a modification of practice and then others that suggest we should look elsewhere for further improvements. With all of this evidence coming out in hundreds if not thousands of journals every week it is difficult to keep up and it may be that our focus is in need of a change in direction or at least devoting members of the team to look at something different.
As the era of the major leaps in care seems to be passing us by what I see more and more are studies looking at how to make further improvements on what we already know. In some cases such as using higher doses of caffeine may reduce the incidence of apnea further compared to standard dosing while cooling for 96 hours instead of 72 and at lower temperatures after asphyxia may not be such a good idea after all. There will be some studies that suggest a modification of practice and then others that suggest we should look elsewhere for further improvements. With all of this evidence coming out in hundreds if not thousands of journals every week it is difficult to keep up and it may be that our focus is in need of a change in direction or at least devoting members of the team to look at something different.
That Focus Is On Quality Improvement (QI)
Before I go on I don’t want to insinuate that I am something that I am not. I do not have any formal training in QI and consider myself an amateur but I do understand enough to undertake a PDSA cycle and see where it takes me. 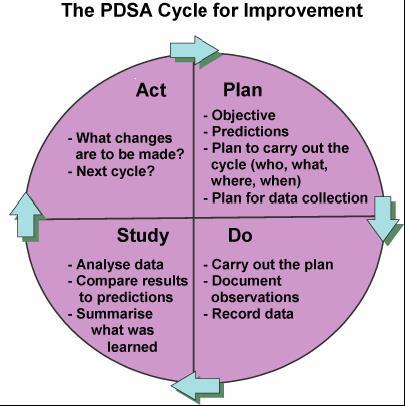 To me QI is about finding ways to actually make your best practices the best they can be. Take for example our units goal to minimize needle pokes by carefully examining the usefulness of common tests that we perform. Add to this the recent implementation of non invasive technology such as transcutaneous bilirubin metres which our evidence suggests can reliably replace a serum sample to screen for those in need of phototherapy. While I commonly like to praise our team for its ability to critically think about needed bloodwork it was only through the collection of data using audit tools that we discovered we had a problem. The problem was that the rate of CBC samples that were clotting were unacceptably high at over 30%. This was compared to another NICU in the same city that had a rate of less than 1/3 that. The initial reaction since it was trained lab personnel collecting at the low clotting site vs nursing at the high rate site was that the solution was simple. Just change back to lab personnel (as it used to be) at the high rate site! Ah but that would create another problem. Other evidence used to build a care plan for our preterm infants suggested that clustering of care was better for them than poking them at the usual run time of the lab techs so we had a conflict. How did we solve it? We resisted our urge for the quick fix and entered into a formal QI project.
To me QI is about finding ways to actually make your best practices the best they can be. Take for example our units goal to minimize needle pokes by carefully examining the usefulness of common tests that we perform. Add to this the recent implementation of non invasive technology such as transcutaneous bilirubin metres which our evidence suggests can reliably replace a serum sample to screen for those in need of phototherapy. While I commonly like to praise our team for its ability to critically think about needed bloodwork it was only through the collection of data using audit tools that we discovered we had a problem. The problem was that the rate of CBC samples that were clotting were unacceptably high at over 30%. This was compared to another NICU in the same city that had a rate of less than 1/3 that. The initial reaction since it was trained lab personnel collecting at the low clotting site vs nursing at the high rate site was that the solution was simple. Just change back to lab personnel (as it used to be) at the high rate site! Ah but that would create another problem. Other evidence used to build a care plan for our preterm infants suggested that clustering of care was better for them than poking them at the usual run time of the lab techs so we had a conflict. How did we solve it? We resisted our urge for the quick fix and entered into a formal QI project.
How did we do it and what were the results?
It took us four rounds of PDSA cycles but in the end we found a solution that has lasted. As I write this I learned that one of our two units that had the high rates set a record low this past month of a 4.9% clotting rate even lower than the comparative site that began with a low clotting rate. It took work and was by no means easy but the dedication of our nurse educator to the task made all the difference. Fortunately, for those who don’t know where to begin an incredible resource is available from BMJ Quality Improvement who provide a step by step process to carry out your project. Moreover after using their template for publishing such work, we were able to publish our work which we hope may be of help for other centres that find themselves in a similar situation. Perhaps the solution might be the same or at least similar enough to try one of our interventions? The full paper can be found at the end of the post but the trend over time is so impressive that I felt obliged to show you the results.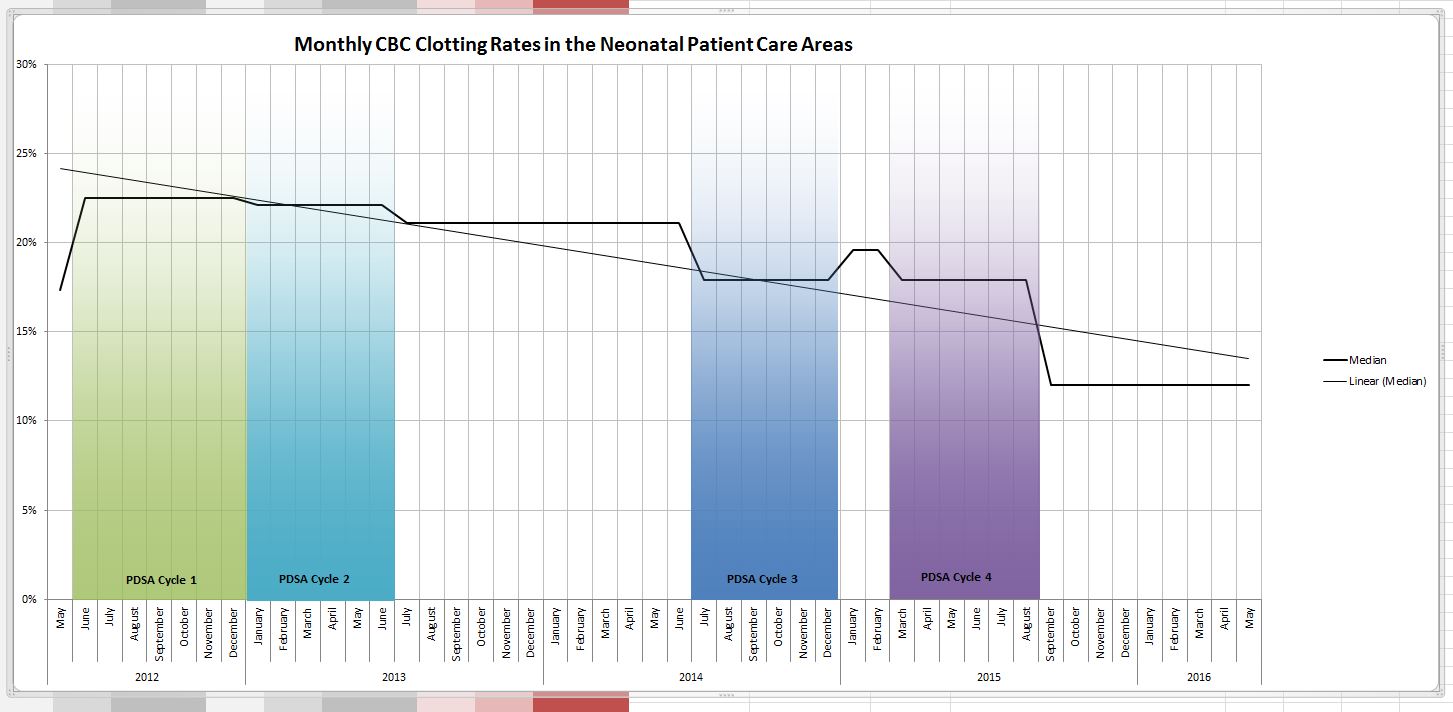
Why should you care?
Teams spend so much time rolling out new evidence based initiatives. All the evidence in world won’t help if the intervention isn’t achieving the results you expect. How will you know unless you audit your results? You may be surprised to find that what you expected in terms of benefit you aren’t seeing. By applying the principals of QI you may find you don’t need to look for another treatment or device but rather you simply need to change your current practice.  A little education and direction may be all that is needed. You may also find to your surprise that what you thought everyone was doing is not what they are doing at all.
A little education and direction may be all that is needed. You may also find to your surprise that what you thought everyone was doing is not what they are doing at all.
Resist the quick solution and put in the time to find the right solution. As Carl Honore suggests slowing things down may be the best thing for all of us and more importantly the patients we care for.
Link to the full article:
References
- Mohammed S et al. High versus low-dose caffeine for apnea of prematurity: a randomized controlled trial. Eur J Pediatr 2015 Jul;174(7):949-56.
- Shankaran S et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial.JAMA 2014 Dec 24-31;312(24):2629-39
by All Things Neonatal | Aug 10, 2016 | Parents, physiotherapy, Uncategorized
I don’t know about your place of work but our centre is busy and by busy I mean our resources and staff are almost always working at full capacity. There is a shift afoot though in modern Neonatal care to shift some of the responsibility for care to the parents. You might say it always should have been this way but as with any speciality we grow, learn and evolve over time.
The most recent stage of evolution is the development of the FiCare philosophy. This is not the first time (and likely not the last) that I will reference this strategy. For more information on what it is and what it takes to practice this concept you can click on the FiCare website from Mt. Sinai Hospital in Toronto here. The gist of it though is that with education and support from nursing in particular some of the traditional functions that are carried out by health care staff can be transferred to the parents. Something as basic as identifying their baby can be a start with progression to providing part of the daily report, participating in handling of their infant during times of stress and performing skin to skin care for many hours a day. The parents are asked to commit to a significant number of hours per day to make this work and the benefits of having close contact are obvious as well.
Can Physiotherapy Be Taught To Parents?
As someone who has been involved in the FiCare project I took particular interest in an article this past month which in essence is related to the teachings of FiCare. T. Ustad from Norway and colleagues published the following Early Parent-Administered Physical Therapy for Preterm Infants: A Randomized Controlled Trial. As someone who values the contribution of our physiotherapists I was curious as to what could be transferred given the demands on an individual PT’s time. Add to this that during surges when many babies under 29 weeks are born and the number of patients they need to see may become overwhelming. What if parents though could take over some of this workload? Well that is what they did in three centres in Norway in a RCT single blinded intervention.
What did they do?
All babies were born < 32 weeks and underwent the intervention between 34 – 36 weeks with final evaluation at 37 week. Parents were taught to perform 10 minutes of manipulation with the goal of improving postural control, head control, and midline orientation. Teaching was done through videos, photos, booklets and direct teaching by the PT on day 1 and then on day 2 return demonstration with correction by the PT occurred.
It was single blinded in that the parent and the PT who did the teaching knew of course which baby received what treatment but the PT doing the standardized evaluation after the two week period was over was blind to treatment assignment. The authors used the TIMP scoring system for spontaneous movements which as it can take up to 30 minutes in some cases may stress the infants so for those a scoring system consisting of half the items was developed called the TIMPSI. This has beee shown previously to correlate well with the larger more involved test.
Sample size calculations were based on finding a difference at 2 years of 0.5 SD between groups which meant they needed 63 infants to show such a difference. They enrolled 153 but after some withdrew the groups were 63 in the intervention and 76 in conventional care.
On to the results
Just so we are clear, this was only designed to be a two week intervention but it was meant to be twice daily for ten minutes at a time. Graphically, using the z-scores for the TIMP scores we have the following graph. Clicking on the highlighted link will tell you more about z-scores but it measures simply how many standard deviations above or below a population mean a score is.
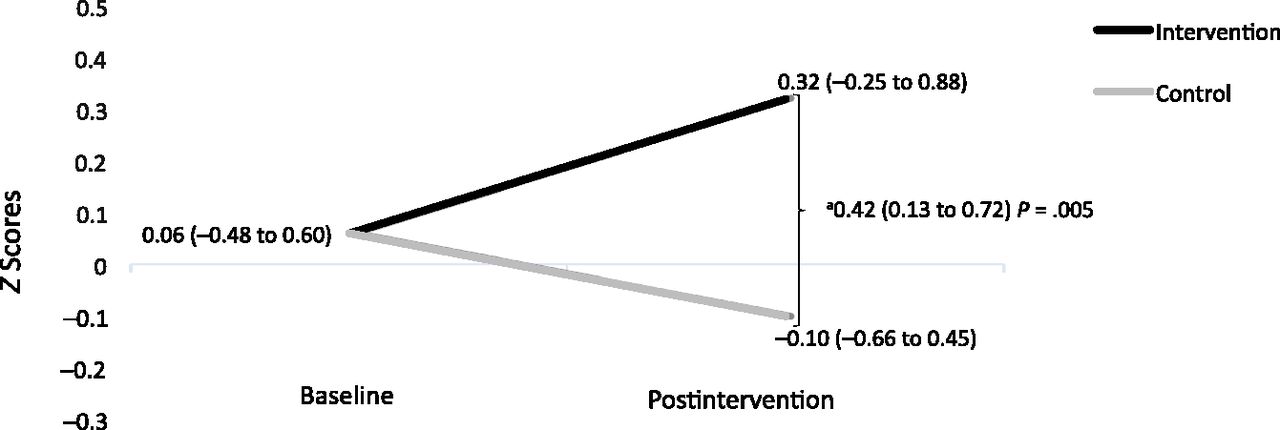
So in this case the absolute difference after the two week period with evaluation one week later is 0.42. This falls short of the 0.5 at two years the sample size calculation was looking for. As with many differences in outcome the results tend to improve with time. Think about the CAP study as an example in which those babies who received caffeine initially had better developmental outcome measures than those who did not but by school age there was no longer a differenced. If the difference is less then one needs many more patients to show a difference than the original sample size would allow. In the end one also needs to think about whether the difference in scores is of statistical interest or if it has true clinical impact.
Some things to consider though
The parents in the intervention group did keep a journal and what they actually did was not what was designed in the study. The average number of sessions per day was only 1.3 with a length of 9 minutes per session. The goals again were 2 for 10 minutes each. The second thing to think about is that by looking at the difference in results from this short intervention it is an exciting mental exercise to think about a couple things. The first is what might the differences look like if the families had been able to do two sessions each per day instead of being closer to 1. The extra minute may not have made such a difference but the extra session might have.
The next thing to consider is how short a time period this really was. What if the plan had been rather than only two weeks, providing the intervention till discharge which for some born at 24 weeks might have been a corrected age at 40 – 44 weeks when they finally went home. Would a much longer exposure have made a bigger difference?
It is always fun to speculate and while I cautioned that the difference seen at two years may narrow further I wonder what the impact on the families will be after the intervention and post discharge. They were taught a new set of techniques to support motor development. Would they simply revert back to the control group afterwards or informally continue on which is what I suspect to some degree they would. The log book doesn’t count the impact of these “extra sessions”. The authors plan an interim analysis along the way so that will be interesting to see.
What the study does show though and what I think is exciting is that it is not just nursing that can transfer some functions to parents. Clearly the parents in this study learned something about handling of patients from the perspective of a PT. I would hope that this study might inspire PTs and other disciplines as the FiCARE approach widens to consider equipping parents with some of their skills sets that are traditionally owned by those specialties alone. What we are discovering with time is that parents are capable of doing more than we have had them do in the past. To make it family centred care truly make them part of the team!






 Here in Canada the majority of the population lives along the border with the US. Take a look though at our population density and you can see that for our Aboriginal (Indian) and Inuit populations as well as all those living in the north we have a significant need to protect them. Living conditions in these places involve overcrowding and high smoking levels both ideal breeding grounds for transmission of RSV and an intolerance to handling the inflammation in your bronchioles. The same is likely true of many parts of Alaska for my US readers.
Here in Canada the majority of the population lives along the border with the US. Take a look though at our population density and you can see that for our Aboriginal (Indian) and Inuit populations as well as all those living in the north we have a significant need to protect them. Living conditions in these places involve overcrowding and high smoking levels both ideal breeding grounds for transmission of RSV and an intolerance to handling the inflammation in your bronchioles. The same is likely true of many parts of Alaska for my US readers.
 As the era of the major leaps in care seems to be passing us by what I see more and more are studies looking at how to make further improvements on what we already know. In some cases such as using
As the era of the major leaps in care seems to be passing us by what I see more and more are studies looking at how to make further improvements on what we already know. In some cases such as using  To me QI is about finding ways to actually make your best practices the best they can be. Take for example our units goal to minimize needle pokes by carefully examining the usefulness of common tests that we perform. Add to this the recent implementation of non invasive technology such as transcutaneous bilirubin metres which our evidence suggests can reliably replace a serum sample to screen for those in need of phototherapy. While I commonly like to praise our team for its ability to critically think about needed bloodwork it was only through the collection of data using audit tools that we discovered we had a problem. The problem was that the rate of CBC samples that were clotting were unacceptably high at over 30%. This was compared to another NICU in the same city that had a rate of less than 1/3 that. The initial reaction since it was trained lab personnel collecting at the low clotting site vs nursing at the high rate site was that the solution was simple. Just change back to lab personnel (as it used to be) at the high rate site! Ah but that would create another problem. Other evidence used to build a care plan for our preterm infants suggested that clustering of care was better for them than poking them at the usual run time of the lab techs so we had a conflict. How did we solve it? We resisted our urge for the quick fix and entered into a formal QI project.
To me QI is about finding ways to actually make your best practices the best they can be. Take for example our units goal to minimize needle pokes by carefully examining the usefulness of common tests that we perform. Add to this the recent implementation of non invasive technology such as transcutaneous bilirubin metres which our evidence suggests can reliably replace a serum sample to screen for those in need of phototherapy. While I commonly like to praise our team for its ability to critically think about needed bloodwork it was only through the collection of data using audit tools that we discovered we had a problem. The problem was that the rate of CBC samples that were clotting were unacceptably high at over 30%. This was compared to another NICU in the same city that had a rate of less than 1/3 that. The initial reaction since it was trained lab personnel collecting at the low clotting site vs nursing at the high rate site was that the solution was simple. Just change back to lab personnel (as it used to be) at the high rate site! Ah but that would create another problem. Other evidence used to build a care plan for our preterm infants suggested that clustering of care was better for them than poking them at the usual run time of the lab techs so we had a conflict. How did we solve it? We resisted our urge for the quick fix and entered into a formal QI project.
 A little education and direction may be all that is needed. You may also find to your surprise that what you thought everyone was doing is not what they are doing at all.
A little education and direction may be all that is needed. You may also find to your surprise that what you thought everyone was doing is not what they are doing at all.
