This is the one as the saying goes that you have all been waiting for! Poractant entered the scene in Canada a few years ago with a lot of promise as a great alternative to the bovine source generally used here. The volume of administration was about half and as the use of MIST/LISA rose in popularity the option to use the lower volume was of interest to many. A study out of London Ontario demonstrated however that the bovine form could be used for LISA/MIST successfully and was written about in Less Invasive Surfactant Administration with High Volume Surfactant.
What about if we look at a real head to head comparison looking at meaningful outcomes like length duration of respiratory support? To do so would require a fairly large sample and would generally be difficult to accomplish but us Canadians opted for a study design to allow this to move forward with a sample size that for a neonatal study I think at least were admirable!
The Study
The study design here was a prospective comparative effectiveness cohort study of babies all born under 32 weeks at 13 NICUs across Canada. The study in question was entitled Poractant alfa versus bovine lipid extract surfactant: prospective comparative effectiveness study and is authored by many I consider colleagues and friends! To do this study each centre agreed to start off for 6 months with the bovine surfactant for any baby that had respiratory distress syndrome and in the opinion of the team needed surfactant. After that period each centre switched to poractant for an additional 6 months. This was a pragmatic trial designed to be less rigid with respect to criteria for intubation and allow for a “real world” determination of effect of using one surfactant vs another. While the study was not randomized the collection of outcome data relied on trained abstractors for the Canadian Neonatal Network in each centre. The authors determined that to see a difference in the primary outcome would require 484 patients per surfactant group. What they obtained in terms of recruitment is shown below.
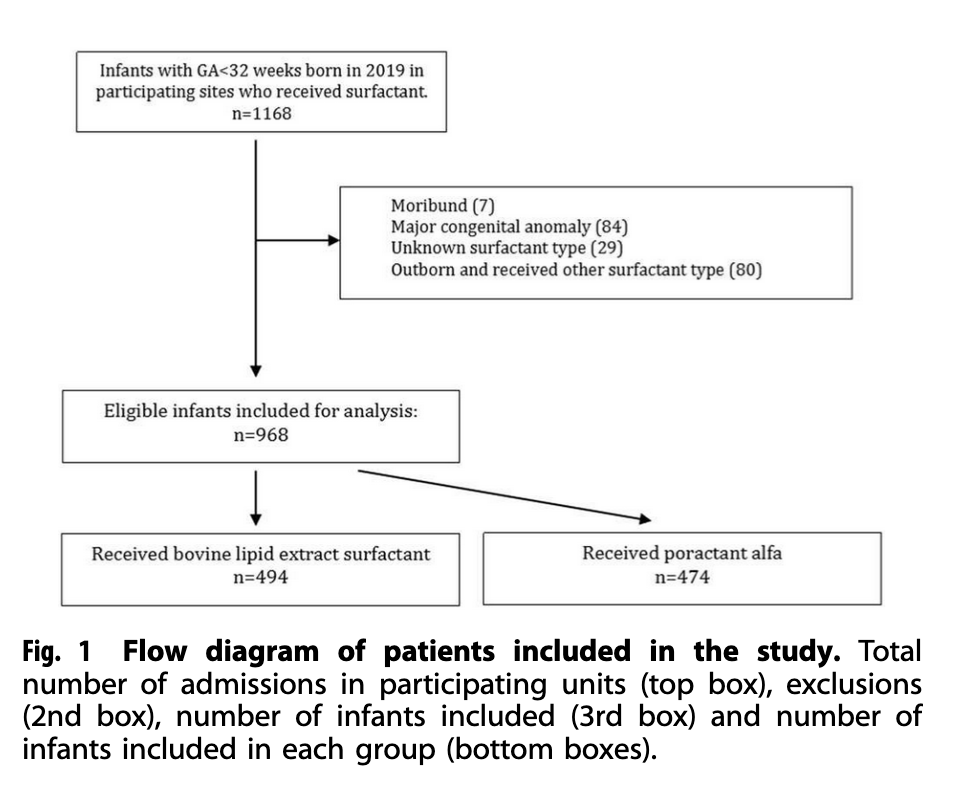
The Results Please
I realize you have been waiting with excitement about what they could have found. Sadly they didn’t find too much!
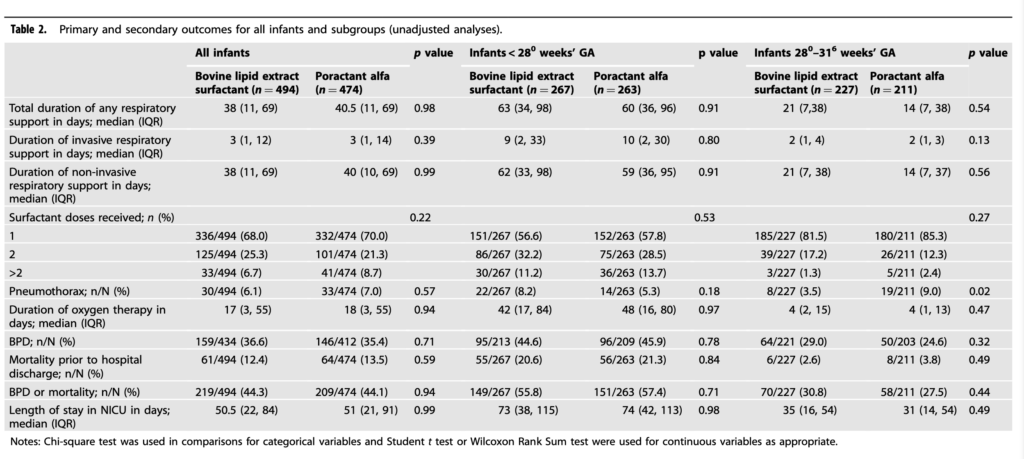
There was no difference in length of ventilation or for that matter some important outcomes like number of doses of surfactant needed (if one group needed more might they be less effective), BPD, mortality and length of stay. The authors did note a difference in rates of MIST/LISA favouring the poractant group but when they controlled for that variable still found no difference in outcomes. Important to note that though since use of MIST/LISA may reduce the outcome of interest itself but alas no difference.
As with many studies people start digging and looking at secondary outcomes to see if there is anything of interest that pops up. It is worth noting here that whatever is found based on this study design would be an association so one must be careful not to jump to causation which may or may not be at play. For fun though let’s look at a couple of things that cropped up.
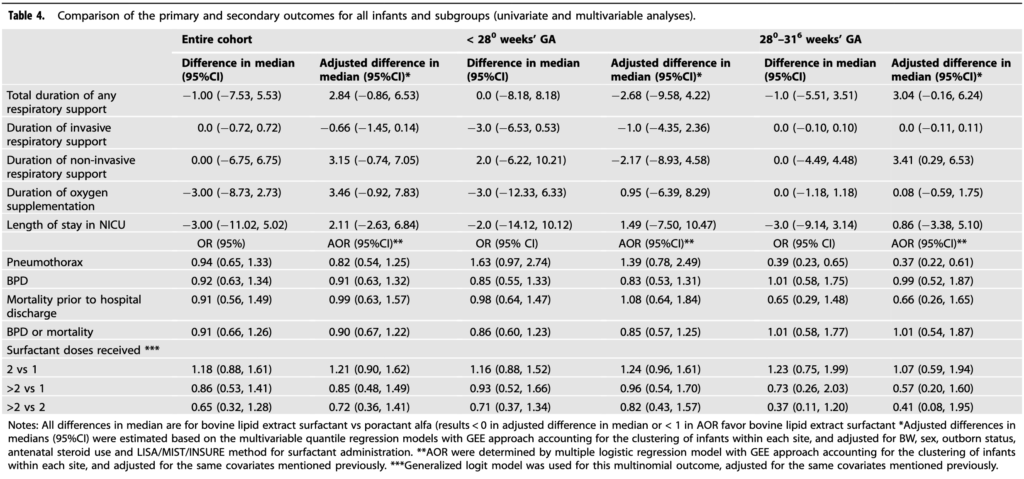
What the study does in my mind is demonstrate that if you wish to use either surfactant you may. I suppose then it comes down to comfort and in part whether you believe that use of a lower volume surfactant is better for administration with MIST/LISA. If that is the case then your choice would be poractant. If you don’t care however then it may come down to cost. There has been a difference in cost but I do wonder if the gap may close with demonstration of similar efficacy in this study. If people are indifferent to utility of the two then cost will certainly be a variable to consider!

Great study. Congratulations!
Based on our experience in my perinatal center ( University maternity hospital in Sofia, Bulgaria) the incidence of pulmonary hemorrhage was higher in intubated preterm infants treated with poractant compared with bovine surfactant.