It gives me great pleasure to write this piece as it is about research that two of my colleagues Dr. Yasser Elsayed and Dr. Shyamala Dakshinamurti authored along with colleagues in Saudi Arabia. Both colleagues have worked in the fields of hemodynamics and pulmonary hypertension for some time. For those of you who know Yasser you would know that he has had an interest in what one can glean from careful attention to a patient monitor for some time. In fact we created some short Youtube videos on these topics a few years ago that are available on the accompanying Youtube channel to this site.
What if you don’t have ready access to an ECHO?
If you are like me, you are blessed to work in a centre that has easy access to evaluation of hemodynamics. What if you are in a centre that doesn’t have such access? Alternatively, in the spirit of using resources wisely what if you don’t want to exhaust your hemodynamics team by asking them to assess every murmur that comes along in a preterm infant. In other words, is there a way to tell whether a ductus is hemodynamically significant or not? There is a lot of preceding research that would tell us that our stethescope and fingertips are not as accurate as we would like to think in determining which ducts are likely significant, if open at all.
In comes the pulsatility index. The pulsatility index is derived from the formula;
(peak systolic velocity – end diastolic velocity) /mean velocity
Moreover, this value can be obtained using an oxygen saturation probe based on the absorption of light by pulsatile and non-pulsatile tissues. Patient monitors can express this number with > 2 being higher than the upper limit of normal in preterm infants, 1–2 being normal, 0.4–1 being low normal, and < 0.4 low PI. The patient monitor moreover is capable of providing histograms for this data providing the user with a bar graph indicating the percentage of time over twenty four hours that the infant has been in each of these ranges. Osman AA et al used this information to study the relationship between PI in the periods of time before an infant develops a HS PDA, during treatment and afterwards in their paper The perfusion index histograms predict patent ductus arteriosus requiring treatment in preterm infants. They studied 34 preterm infants in four time periods, namely 24 h before starting treatment of PDA, during PDA treatment, and 24 h after completion of the course of treatment, and confirmed PDA closure by echo. The data was obtained from a oxygen saturation probe placed on the right wrist in a preductal location. They also compared PI during matched time periods in infants without a HS PDA in order to determine what the PI ranges would be for patients of a matched gestational age without a ductal concern.
For the ECHO diagnosis of a HS PDA they used the following criteria:
A ductal diameter at the pulmonary side ≥ 1.5 mm and at least one of the following:
- left atrial to aortic ratio ≥ 1.5
- Left ventricular output > 300 ml/kg/min
- PDA peak systolic velocity < 1.5 m/s
- PDA peak systolic velocity/minimum diastolic velocity > 4
What did they find?
Before the PDA was treated but was identified as being significant the figure below shows that the incidence of low flows were statistically more likely to be present ini HS PDA. This remained true during treatment with a stabilization follwoing treatment.
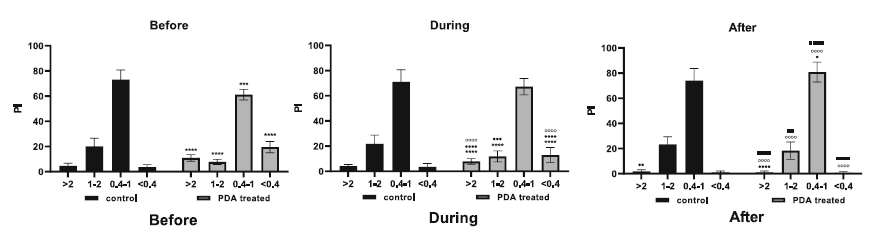
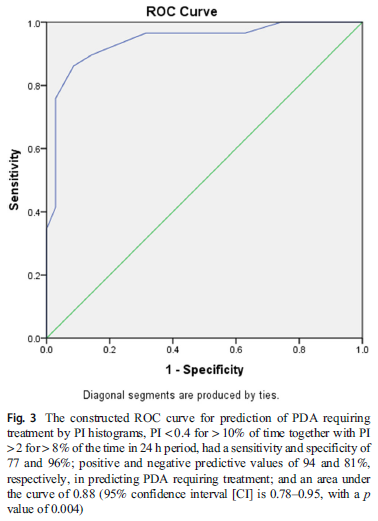
The authors examined the best predictive findings from the histogram analysis and discovered that “presence of a PI <0.4 for > 10% of the recorded time, together with the presence of a PI > 2 for > 8% of the time recorded, is predictive of a PDA requiring treatment, with a sensitivity and specificity of 77 and 96%; positive and negative predictive values of 94 and 81%, respectively;and an area under the curve of 0.88 (95% confidence interval 0.78–0.95, p = 0.004)”
How do we explain this in terms of physiology? The low flow state I think is the easier one to think about. If you have a HS PDA blood is “stolen” from the aorta and is directed to the lungs. This stolen flow may lead to lower perfusion than normal to the distal extremities as aortic flow is less than what it normally would be. Therefore with less flow in a vessel the pulsatility index declines. How then could you find a high PI in the same patient? The situation may arise if there is intermittent hypoxia that increases pulmonary vascular resistance thereby lessening the flow and restoring good flow in the aorta. The combination therefore was found to be predictive of a HS PDA with a reasonable specificity in particular. If you don’t have a low PI it is unlikely you have a HS PDA.
How could we use this?
Don’t worry I am not going to suggest that we can do away with the hemodynamics assessment. I do wonder though if this information could be very useful in helping to triage resources when they may be quite limited. In other words, if you hear on morning report that a 27 week infant has become tachypneic and has a murmur, instead of jumping to call the Hemodynamics service why not check the 24 hour PI histogram? If you don’t see low flows it is unlikely as I read this that a HS PDA is present. To be clear I am not saying that I am totally sold on this! I think it needs to be recognized this was a small study and will need further larger samples to confirm as there would be more babies with varying levels of PDAs in terms of hemodynamics to study. In the meantime though I think it would be very interesting to take a look at the 24 hour PI histograms for the next number of babies I see and look at how it does correlate with the ECHO I ask for. No doubt as two of the authors in the paper work with me I won’t have to remember this post to check the values as I am sure this is not the last I will hear of this!


I saw twins by echo yesterday, previously momo-mono delivered at 33 weeks, now off respiratory support, both with O2 sat = 100% and nearly identical weights. Both had heart murmurs inn the manubrial-left clavicular area. Twin A BP was 68/39 which was average for the baby over 3 days. Twin B BP was 78/28 which was average for the baby over 3 days. Twin A had a 1mm PDA, peak velocity of 4M continuously while twin B had LPA acceleration over 2 M, which we call LPPS. The LV size and function in each were identical with the only difference a mildly dilated LA:Ao in the A. Of course we teach widening pulse pressure in an HS PDA as the PAP falls. But there are always exceptions