Neurally adjusted ventilatory assistance or NAVA is something that has been around for awhile. Available as a mode on the Maquet ventilator it uses an esophageal probe to sense myoelectrical activity in the diaphragm and provide assistance with postive pressure when detected. This is supposed to be better than the more traditional Graseby capsules or sensing based on airflow. Conceptually then if a preterm infant had a typical mixed apneic event with a component of both central and obstructive apnea this technology could sense an attempt to breath and assist the infant with positive pressure when the diaphragm indicates it is time for a breath. Such support should work to maintain functional residual capacity. A better ventilated lung could lead to less systemic oxygen desaturation and bradycardia correct?
Retrospective review in Virginia
Tabacaru CR et al just published NAVA—synchronized compared to nonsynchronized noninvasive ventilation for apnea, bradycardia, and desaturation events in VLBW infants. This is a retrospective study and non randomized looking at a single centres experience in 108 VLBW infants in which the attending providers were free to choose the type of respiratory support infants received after extubation. The authors from this group examined 61 epochs of time on niNAVA compared to 103 for the non invasive positive pressue ventilation nIPPV group. niNAVA patients received an initial level (the factor by which the electric diaphragmatic signal intensity (edi) is multiplied) of 1.0 and a PEEP of 5 to 6 cm H2O. NIPPV was initiated at a positive inspiratory prrssure (PI)P of 14 to 16 cm H2O, PEEP of 5 to 6 cm H2O and a rate of 20 breaths per minute. Adjustments were dictated by oxygenation and blood gases and were not described as protocolized but rather left up to clinicians. All events were recorded manually by nursing.
What impact did niNAVA have on apnea and bradycardia?
There were no significant differences noted between the two study groups including such important parameters as birthweight, day of life of extubation, sepsis or whether they needed to be reintubated. All of these could be markers of worse lungs in one group or the other so at least them seem pretty much the same.
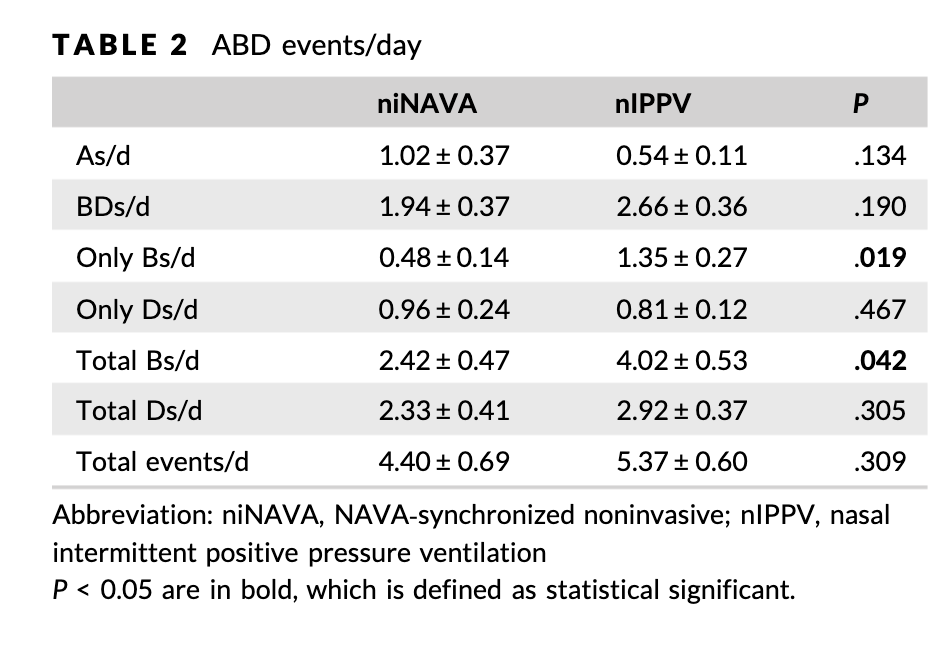
What about the effect on apnea and bradycardia? The bold numbers in the table indicate that only the number of bradycardias per day differed between the groups. Whether patients desaturation events or not was not affected. Also not effected was whether or not patients had apnea.
Why might these results make sense?
First off since the study was not randomized and is small there is always the possibility that these results are not real and occurred just by chance. There could be variables for example that we are not taking into account to explain why some patients were chosen for one modality or the other than affect the outcomes here. Having said that let’s look at the three outcomes.
- Apnea – why would this be different at all? Both modalities provide support when needed. If the infant decides to stop breathing I would see the lack of neural output not being affected by either modality so perhaps if the primary issue is lack of respiratory drive for most we wouldn’t expect a difference.
- Desaturation – if pulmonary reserve is kept about the same with both approaches it seems reasonable that we might not see a difference here either.
- Bradycardia – here there was a difference. Can this be explained as something plausible. I think there might be something here. Use of NAVA just might have a faster and more accurate response time than nIPPV that relies on airflow. Due to leaks around the prongs or mask it is possible that while background pressures are relatively maintained, not all needed positive pressure helping breaths are received in as timely a fashion as when they are detected via electrical activity. The ability of niNAVA to help the infant overcome the obstructive component of breathing might be reason why bradycardia is reduced. The interruption of ventilation is briefer with less reflexive bradycardia.
What is needed of course next is a randomized prospective controlled trial. Who knows when that will come but for the infants that we see with seeminly methylxanthine resistant apnea might niNAVA be the path to avoiding reintubations? Time will tell

