
by All Things Neonatal | Aug 15, 2018 | intubation, Neonatal, Neonatology, technology
The modern NICU is one that is full of patients on CPAP these days. As I have mentioned before, the opportunity to intubate is therefore becoming more and more rare is non-invasive pressure support becomes the mainstay of therapy. Even for those with established skills in placing an endotracheal tube, the number of times one gets to do this per year is certainly becoming fewer and fewer. Coming to the rescue is the promise of easier intubations by being able to visualize an airway on a screen using a video laryngoscope. The advantage to the user is that anyone who is watching can give you some great tips and armed with this knowledge you may be better able to determine how to adjust your approach.
For those of you who have followed the blog for some time, you will recall this is not the first time video laryngoscopy has come up. I have spoken about this before in Can Video Laryngoscopy Improve Trainee Success in Intubation. In that piece, the case was made that training residents how to intubate using a video laryngoscope (VL) improves their success rate. An additional question that one might ask though has to do with the quality of the intubation. What if you can place a tube using a video laryngoscope but the patient suffers in some way from having that piece of equipment in the mouth? Lucky for us some researchers from the Children’s Hospital of Philadelphia have completed a study that can help answer this additional question.
Video Laryngoscopy may work but does it cause more harm than good?
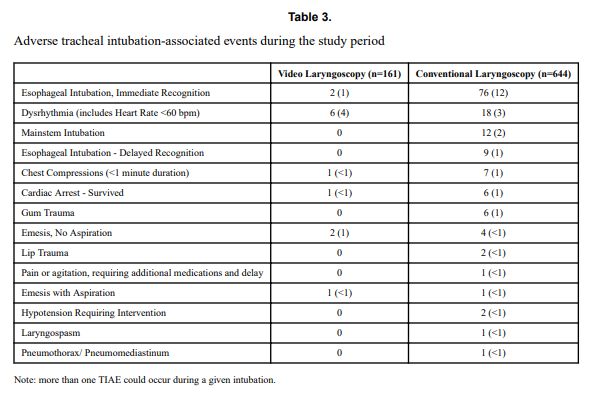
Using a video laryngoscope requires purchasing one first and they aren’t necessarily cheap. If they were to provide a better patient experience though the added cost might well be worth it. Pouppirt NR et al published Association Between Video Laryngoscopy and Adverse Tracheal Intubation-Associated Events in the Neonatal Care Unit. This study was a retrospective comparison of two groups; one having an intubation performed with a VL (n=161 or 20% of the group) and the other with a standard laryngoscope (644 or 80% of the group). The study relied on the use of the National Emergency Airway Registry for Neonates (NEAR4NEOs), which records all intubations from a number of centres using an online database and allows for analysis of many different aspects of intubations in neonates. In this case the data utilized though was from their centre only to minimize variation in premedication and practitioner experience.
Tracheal intubation adverse events (TIAEs) were subdivided into severe (cardiac arrest, esophageal intubation with delayed recognition, emesis with witnessed aspiration, hypotension requiring intervention (fluid and/or vasopressors), laryngospasm, malignant hyperthermia, pneumothorax/pneumomediastinum, or direct airway injury) vs non-severe (mainstem bronchial intubation, esophageal intubation with immediate recognition, emesis without aspiration, hypertension requiring therapy, epistaxis, lip trauma, gum or oral trauma, dysrhythmia, and pain and/or agitation requiring additional medication and causing a delay in intubation.
Looking at the patient characteristics and outcomes, some interesting findings emerge.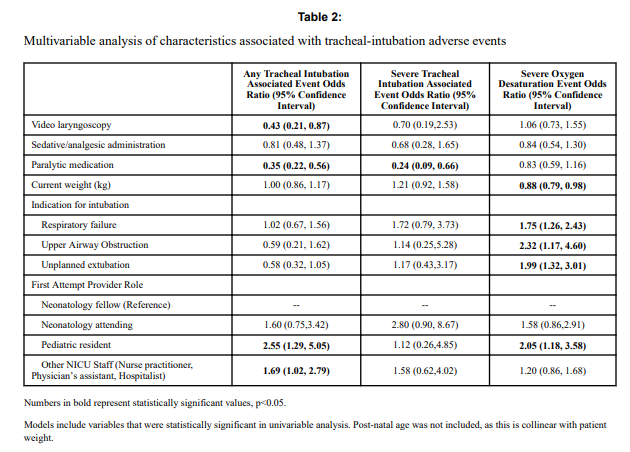

Patients who had the use of the VL were older and weighed more. They were more likely to have the VL used for airway obstruction than respiratory failure and importantly were also more likely to receive sedation/analgesia and paralysis. These researchers have also recently shown that the use of paralysis is associated with less TIAEs so one needs to bear this in mind when looking at the rates of TIAEs. There were a statistically significant difference in TIAEs of any type of 6% in the VL group to 19% in the traditional laryngoscopy arm but severe TIAEs showed not difference.
Given that several of the baseline characteristics might play a role in explaining why VL seemed superior in terms of minimizing risk of TIAEs by two thirds, the authors performed a multivariable analysis in which they took all factors that were different into account and then looked to see if there was still an effect of the VL despite these seemingly important differences. Interestingly, use of VL showed an Odds ratio of 0.43 (0.21,0.87 95% CI) in spite of these differences.
What does it mean?
Video laryngoscopy appears to make a difference to reducing the risk on TIAEs as an independent factor. The most common TIAE was esophageal intubation at 10% and reducing that is a good thing as it leads to fewer intubation attempts. This was also sen as the first attempt success was 63% in the VL group vs 44% in the other.
Now we need to acknowledge that this was not a randomized controlled trial so it could indeed be that there are other factors that the authors have not identified that led to improvements in TIAEs as well. What makes this study so robust though is the rigour with which the centre documents all of their intubations using such a detailed registry. By using one centre much of the variability in practice between units is eliminated so perhaps these results can be trusted. Would your centre achieve these same results? Maybe not but it would certainly be interesting to test drive one of these for a period of time see how it performs.

by All Things Neonatal | Jun 30, 2018 | intubation, Neonatal, Neonatology, newborn, preemie, Prematurity
A few weeks back I wrote about the topic of intubations and whether premedication is really needed (Still performing awake intubations in newborns? Maybe this will change your mind.) I was clear in my belief that it is and offered reasons why. There is another group of practitioners though that generally agree that premedication is beneficial but have a different question. Many believe that analgesia or sedation is needed but question the need for paralysis. The usual argument is that if the intubation doesn’t go well and the patient can’t spontaneously ventilate could we be worse off if the patient loses their muscle tone.
Neonatal Intubation Registry
At the CPS meeting last month in Quebec City. I had the pleasure of listening to a talk by Dr. Elizabeth Foglia on the findings from a Neonatal intubation registry that many centres have been contributing to. The National Emergency Airway Registry for Neonates (NEAR4NEOs), records all intubations from a number of centres using an online database and allows for analysis of many different aspects of intubations in neonates.
This year, J. Krick et al published Premedication with paralysis improves intubation success and decreases adverse events in very low birth weight infants: a prospective cohort study. This study compared results from the registry of two centres, the University of Washington Medical Center (UWMC) and Seattle Children’s Hospital where the former rarely uses paralysis and the latter in almost all instances of non-emergent intubation. In all, 237 encounters were analyzed in the NICU for babies < 1500g with the majority of encounters (181) being from UWMC. The median PMA at intubation was 28 completed weeks (IQR: 27, 30), chronological age was 9 days (IQR: 2, 26) and weight was 953 g (IQR: 742,1200). The babies were compared based on the following groups. Premedication with a paralytic 21%, without a paralytic 46% and no premedication 31%.
This was an observational study that examined the rates of adverse events and subdivided into severe (cardiac arrest, esophageal intubation with delayed recognition, emesis with witnessed aspiration, hypotension requiring intervention (fluid and/or vasopressors), laryngospasm, malignant hyperthermia, pneumothorax/pneumomediastinum, or direct airway injury) vs non-severe (mainstem bronchial intuba- tion, esophageal intubation with immediate recognition, emesis without aspiration, hypertension requiring therapy, epistaxis, lip trauma, gum or oral trauma, dysrhythmia, and pain and/or agitation requiring additional medication and causing a delay in intubation.).
How did the groups compare?
It turns out paralysis seems to be a big deal (at least in this group of infants). Use of paralysis resulted in less attempts to intubate (median 1 attempt; IQR: 1, 2.25 vs. 2; IQR: 1, 3, p < 0.05)). In fact success was no different between the groups with no paralysis or no premedication at all! When it comes to tracheal intubation adverse events the impact of using paralysis becomes more evident. 
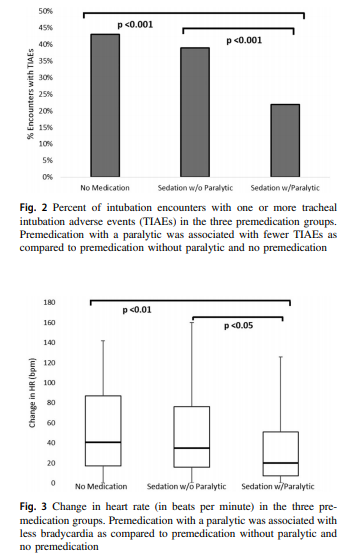 Paralysis does make a difference in reducing the incidence of such events and moreover when only looking at the rate of severe adverse events as defined above the finding was that none occurred when paralysis was used vs 9 when no paralysis was employed and 5 when no premedication was used at all. The rate of bradycardic events was less in the paralytic group but rates of oxygen desaturation between the three arms were no different.
Paralysis does make a difference in reducing the incidence of such events and moreover when only looking at the rate of severe adverse events as defined above the finding was that none occurred when paralysis was used vs 9 when no paralysis was employed and 5 when no premedication was used at all. The rate of bradycardic events was less in the paralytic group but rates of oxygen desaturation between the three arms were no different.
How do we interpret the results?
Based on the results from the registry it looks like paralysis is a good thing here when electively intubating infants. If we try to determine the reason for it I suspect it may have much to do with the higher likelihood of success on the first attempt at placing an ETT. The longer it takes to place the ETT or the more number of attempts requiring intermittent PPV in a patient who truly needs a tube the greater the likelihood that you will see adverse events including bradycardia. It may simply be that a calm and still patient is an easier intubation and getting the tube in faster yields a more stable patient.
I am biased though and I think it is worth pointing out another possible reason for the differing results. One hospital in this study routinely used premedication and the other did not. Almost 3/4 of the patients came from one hospital which raises the possibility that skill set could be playing a role. If the skill of providers at the two hospitals differed, the results could reflect the variable skill in the practitioners versus the difference in the medications used themselves. What I don’t know though is whether the two share the same training program or not. Are the trainees the same at both sites (google maps says the two sites are 11 minutes away by car)? The difference still might be in local respiratory therapists or Neonatologists intubating as well. Regardless, the study provides evidence that paralysis makes a difference. To convince those out there though who remain skeptical I think we are going to need the registry to take part in a prospective trial using many centres. A format in which several centres that don’t use paralysis are compared to several who do routinely would help to sort out the concern in skill when looking only at two centres. This wouldn’t be randomized of course but I think it would be very difficult at this point to get a centre that strongly believes in using paralysis to randomize so a prospective study using groups chosen by the individual centre might be the next best thing. If anyone using the registry is reading this let me know what you think?
by All Things Neonatal | May 30, 2018 | intubation, Neonatal, Neonatology, preemie, Prematurity, resuscitation
If I look back on my career there have been many things I have been passionate about but the one that sticks out as the most longstanding is premedicating newborns prior to non-emergent intubation. The bolded words in the last sentence are meant to reinforce that in the setting of a newborn who is deteriorating rapidly it would be inappropriate to wait for medications to be drawn up if the infant is already experiencing severe oxygen desaturation and/or bradycardia. The CPS Fetus and Newborn committee of which I am a member has a statement on the use of premedication which seems as relevant today as when it was first developed. In this statement the suggested cocktail of atropine, fentanyl and succinylcholine is recommended and having used it in our centre I can confirm that it is effective. In spite of this recommendation by our national organization there remain those who are skeptical of the need for this altogether and then there are others who continue to search for a better cocktail. Since I am at the annual conference for the CPS in Quebec city  I thought it would be appropriate to provide a few comments on this topic.
I thought it would be appropriate to provide a few comments on this topic.
Three concerns with rapid sequence induction (RSI) for premedication before intubation
1. “I don’t need it. I don’t have any trouble intubating a newborn” – This is perhaps the most common reason I hear naysayers raise. There is no question that an 60-90 kg practitioner can overpower a < 5kg infant and in particular an ELBW infant weighing < 1 kg. This misses the point though. Premedicating has been shown to increase success on the first attempt and shorten times to intubation. Dempsey 2006, Roberts 2006, Carbajal 2007, Lemyre 2009
2. “I usually get in on the first attempt and am very slick so risk of injury is less.” Not really true overall. No doubt there are those individuals who are highly successful but overall the risk of adverse events is reduced with premedication. (Marshall 1984, Lemyre 2009). I would also proudly add another Canadian study from Edmonton by Dr. Byrne and Dr. Barrington who performed 249 consecutive intubations with predication and noted minimal side effects but high success rates at first pass.
3. “Intubation is not a painful procedure”. This one is somewhat tough to obtain a true answer for as the neonate of course cannot speak to this. There is evidence available again from Canadian colleagues in 1984 and 1989 that would suggest that infants at the very least experience discomfort or show physiologic signs of stress when intubated using an “awake” approach. In 1984 Kelly and Finer in Edmonton published Nasotracheal intubation in the neonate: physiologic responses and effects of atropine and pancuronium. This randomized study of atropine with or without pancuronium vs control demonstrated intracranial hypertension only in those infants in the control arm with premedication ameliorating this finding. Similarly, in 1989 Barrington, Finer and the late Phil Etches also in Edmonton published Succinylcholine and atropine for premedication of the newborn infant before nasotracheal intubation: a randomized, controlled trial. This small study of 20 infants demonstrated the same finding of elimination of intracranial hypertension with premedication. At the very least I would suggest that having a laryngoscope blade put in your oral cavity while awake must be uncomfortable. If you still doubt that statement ask yourself whether you would want sedation if you needed to be intubated? Still feel the same way about babies not needing any?
4. What if I sedate and paralyze and there is a critical airway? Well this one may be something to consider. If one knows there is a large mass such as a cystic hygroma it may be best to leave the sedation or at least the paralysis out. The concern though that there might be an internal mass or obstruction that we just don’t know about seems a little unfounded as a justification for avoiding medications though.
Do we have the right cocktail?
The short answer is “I don’t know”. What I do know is that the use of atropine, an opioid and a muscle relaxant seems to provide good conditions for intubating newborns. We are in the era of refinement though and as a recent paper suggests, there could be alternatives to consider;Effect of Atropine With Propofol vs Atropine With Atracurium and Sufentanil on Oxygen Desaturation in Neonates Requiring Nonemergency IntubationA Randomized Clinical Trial. I personally like the idea of a two drug combination for intubating vs.. three as it leaves one less drug to worry about a medication error with. There are many papers out there looking at different drug combinations. This one though didn’t find a difference between the two combinations in terms of prolonged desaturations between the two groups which was the primary outcome. Interestingly though the process of intubating was longer with atropine and propofol. Given some peoples reluctance to use RSI at all, any drug combination which adds time to the the procedure is unlikely to go over well. Stay tuned though as I am sure there will be many other combinations over the next few years to try out!

by All Things Neonatal | Apr 1, 2018 | Neonatal, Neonatology, preemie, Prematurity, ventilation
For almost a decade now confirmation of intubation is to be done using detection of exhaled CO2. The 7th Edition of NRP has the following to say about confirmation of ETT placement “The primary methods of confirming endotracheal tube placement within the trachea are detecting exhaled CO2 and a rapidly rising heart rate.” They further acknowledge that there are two options for determining the presence of CO2 “There are 2 types of CO2 detectors available. Colorimetric devices change color in the presence of CO2. These are the most commonly used devices in the delivery room. Capnographs are electronic monitors that display the CO2 concentration with each breath.” The NRP program stops short of recommending one versus the other. I don’t have access to the costs of the colorimetric detectors but I would imagine they are MUCH cheaper than the equipment and sensors required to perform capnography using the NM3 monitor as an example. The real question though is if capnography is truly better and might change practice and create a safer resuscitation, is it the way to go?
Fast but not fast enough?
So we have a direct comparison to look at. Hunt KA st al published Detection of exhaled carbon dioxide following intubation during resuscitation at delivery this month. They started from the standpoint of knowing from the manufacturer of the Pedicap that it takes a partial pressure of CO2 of 4 mm Hg to begin seeing a colour change from purple to yellow but only when the CO2 reaches 15 mm Hg do you see a consistent colour change with that device. The capnograph from the NM3 monitor on the other hand is quantitative so is able to accurately display when those two thresholds are reached. This allowed the group to compare how long it took to see the first colour change compared to any detection of CO2 and then at the 4 and 15 mm Hg levels to see which is the quicker method of detection. It is an interesting question as what would happen if you were in a resuscitation and the person intubates and swears that they are in but there is no colour change for 5, 10 or 15 seconds or longer? At what point do you pull the ETT? Compare that with a quantitative method in which there is CO2 present but it is lower than 4. Would you leave the tube in and use more pressure (either PIP/PEEP or both?)? Before looking at the results, it will not shock you that ANY CO2 should be detected faster than two thresholds but does it make a difference to your resuscitation?
The Head to Head Comparison
The study was done retrospectively for 64 infants with a confirmed intubation using the NM3 monitor and capnography. Notably the centre did not use a colorimetric detector as a comparison group but rather relied on the manufacturers data indicating the 4 and 15 mm Hg thresholds for colour changes. The mean age of patients intubated was 27 weeks with a range of 23 – 34 weeks. The results I believe show something quite interesting and informative.
|
Median time secs (range) |
| Earliest CO2 detection |
3.7 (0 – 44s) |
| 4 mm Hg |
5.3 (0 – 727) |
| 15 mm Hg |
8.1 (0 – 727) |
I wouldn’t worry too much about a difference of 1.6 seconds to start getting a colour change but it is the range that has me a little worried. The vast majority of the patients demonstrated a level of 4 or 15 mm Hg within 50 seconds although many were found to take 25-50 seconds. When compared to a highest level of 44 seconds in the first detection of CO2 group it leads one to scratch their head. How many times have you been in a resuscitation and with no CO2 change you keep the ETT in past 25 seconds? Looking closer at the patients, there were 12 patients that took more than 30 seconds to reach a threshold of 4 mm Hg. All but one of the patients had a heart rate in between 60-85. Additionally there was an inverse relationship found between gestational age and time to detection. In other words, the smallest of the babies in the study took the longest to establish the threshold of 4 and 15 mm Hg.
Putting it into context?
What this study tells me is that the most fragile of infants may take the longest time to register a colour change using the colorimetric devices. It may well be that these infants take longer to open up their pulmonary vasculature and deliver CO2 to the alveoli. As well these same infants may take longer to open the lung and exhale the CO2. I suppose I worry that when a resuscitation is not going well and an infant at 25 weeks is bradycardic and being given PPV through an ETT without colour change, are they really not intubated? In our own centre we use capnometry in these infants (looks for a wave form of CO2) which may be the best option if you are looking to avoid purchasing equipment for quantitative CO2 measurements. I do worry though that in places where the colorimetric devices are used for all there will be patients who are extubated due to the thought that they in fact have an esophageal intubation when the truth is they just need time to get the CO2 high enough to register a change in colour.
Anyways, this is food for thought and a chance to look at your own practice and see if it is in need of a tweak…

by All Things Neonatal | Feb 16, 2018 | intubation, surfactant
A common concern in the NICU these days is the lack of opportunity to intubate. A combination of an increasing pool of learners combined with a move towards a greater reliance on non-invasive means of respiratory support is to blame in large part. With this trend comes a declining opportunity to practice this important skill and with it a challenge to get a tube into the trachea when it really counts. One such situation is a baby with escalating FiO2 requirements who one wishes to provide surfactant to. Work continues to be done in the area of aerosolized surfactant but as of yet this is not quite ready for prime time. What if there was another way to get surfactant to where it was needed without having to instill it directly into the trachea whether through a catheter (using minimally invasive techniques) or through an endotracheal tube?
Installation of surfactant into the trachea
Lamberska T et al have published an interesting pilot study looking at this exact strategy. Their paper entitled Oropharyngeal surfactant can improve initial stabilisation and reduce rescue intubation in infants born below 25 weeks of gestation takes a look at a strategy of instilling 1.5 mL of curosurf directly into the pharynx for infants 22-24 weeks through a catheter inserted 3-4 cm past the lips as a rapid bolus concurrent with a sustained inflation maneuver (SIM) of 25 cm of H2O for 15 seconds. Two more SIMs were allowed of the heart rate remained < 100 after 15 seconds of SIM. The theory here was that the SIM would trigger an aspiration reflex as the pressure in the pharynx increased leading to distribution of surfactant to the lung. The study compared three epochs from January 2011 – December 2012 when SIM was not generally practiced to July 2014 – December 2015 when SIM was obligatory. The actual study group was the period in between when prophylactic surfactant with SIM was practiced for 19 infants.
A strength of the study was that resuscitation practices were fairly standard outside of these changes in practice immediately after delivery and the decision to intubate if the FiO2 was persistently above 30% for infants on CPAP. A weakness is the size of the study with only 19 patients receiving this technique being compared to 20 patients before and 20 after that period. Not very big and secondly no blinding was used so when looking at respiratory outcomes one has to be careful to ensure that no bias may have crept in. If the researchers were strongly hoping for an effect might they ignore some of the “rules around intubation” and allow FiO2 to creep a little higher on CPAP as an example? Hard to say but a risk with this type of study.
What did they find?
The patients in the three epochs were no different from one and other with one potentially important exception. There were higher rates of antenatal steroid use in the study group (95% vs 75 and 80% in the pre and post study epochs). Given the effect of antenatal steroids on reducing respiratory morbidity, this cannot be ignored and written off.
Despite this difference it is hard to ignore the difference in endotracheal intubation in the delivery room with only 16% needing this in the study group vs 75 and 55% in the other two time periods. Interestingly, all of the babies intubated in the delivery area received surfactant at the same percentages as above. The need for surfactant in the NICU however was much higher in the study period with 79% receiving a dose in the study group vs 20 and 35% in the pre and post study groups. Other outcomes such as IVH, severe ROP and BPD were looked at with no differences but the sample again was small.
What can we take from this?
Even taking into account the effect of antenatal steroids, I would surmise that some surfactant did indeed get into the trachea of the infants in the study group. This likely explains the temporary benefit the babies had in the delivery suite. I suspect that there simply was not a big enough dose to fully treat their RDS leading to eventual failure on CPAP and a requirement for intubation. Is all lost though? Not really I think. Imagine you are in a centre where the Neonatologist is not in house and while he/she is called to the delivery they just don’t make it in time. The trainee tries to intubate but can’t get the tube in. Rather than trying several times and causing significant amounts of airway trauma (as well as trauma to their own self confidence) they could abandon further attempts and try instilling some surfactant into the pharynx and proving a SIM. If it works at all the baby might improve enough to buy some time for them to be stabilized on CPAP allowing time for another intubater to arrive.
While I don’t think there is enough here to recommend this as an everyday practice there just might be enough to use this when the going gets tough. No doubt a larger study will reveal whether there really is something here to incorporate into the tool chest that we use to save the lives of our smallest infants.







 Paralysis does make a difference in reducing the incidence of such events and moreover when only looking at the rate of severe adverse events as defined above the finding was that none occurred when paralysis was used vs 9 when no paralysis was employed and 5 when no premedication was used at all. The rate of bradycardic events was less in the paralytic group but rates of oxygen desaturation between the three arms were no different.
Paralysis does make a difference in reducing the incidence of such events and moreover when only looking at the rate of severe adverse events as defined above the finding was that none occurred when paralysis was used vs 9 when no paralysis was employed and 5 when no premedication was used at all. The rate of bradycardic events was less in the paralytic group but rates of oxygen desaturation between the three arms were no different.
 I thought it would be appropriate to provide a few comments on this topic.
I thought it would be appropriate to provide a few comments on this topic.