
by All Things Neonatal | Jun 27, 2018 | NAS, Neonatal, neonatal abstinence, Neonatology
 This post is very timely as the CPS Fetus and Newborn committee has just released a new practice point:
This post is very timely as the CPS Fetus and Newborn committee has just released a new practice point:
Managing infants born to mothers who have used opioids during pregnancy
Have a look at discharge considerations as that section in the statement speaks to this topic as well!
As bed pressures mount seemingly everywhere and “patient flow” becomes the catch-word of the day, wouldn’t it be nice to manage NAS patients in their homes? In many centres, such patients if hospitalized can take up to 3 weeks on average to discharge home off medications. Although done sporadically in our own centre, the question remains is one approach better than the another? Nothing is ever simple though and no doubt there are many factors to consider depending on where you live and what resources are available to you. Do you have outpatient follow-up at your disposal with practitioners well versed in the symptoms of NAS and moreover know what to do about them? Is there comfort in the first place with sending babies home on an opioid or phenobarbital with potential side effects of sedation and poor feeding? Nonetheless, the temptation to shift therapy from an inpatient to outpatient approach is very tempting.
The Tennessee Experience
Maalouf Fl et al have published an interesting account of the experience with outpatient therapy in their paper Outpatient Pharmacotherapy for Neonatal Abstinence Syndrome. The authors were able to take advantage of the Tennessee Medicaid program using administrative
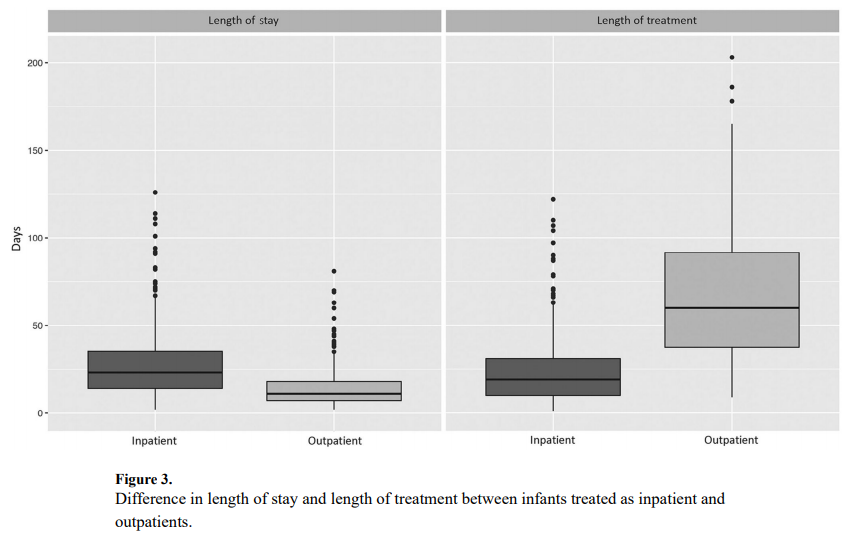
and vital records data from 2009 to 2011 to capture a cohort of 736 patients who were treated for NAS. Forty five percent or 242 patients were treated as outpatients vs 290 cared for in hospital for the duration of treatment. It is worth mentioning at this point that when the authors say they were cared for as outpatients it really is a hybrid model as the duration of hospitalization for the inpatients was a median of 23 days (IQR 14-35) versus 11 days (IQR 7-18) for inpatients (P < .001). This practice isn’t much different than my own in which I start therapy in hospital and then discharge home with a period of home therapy.
The strength of the study is the volume of patients and the ability to follow-up with these babies for the first 6 months of life to determine what happened to them after discharge. In terms of duration of treatment, the differences are significant but perhaps not surprising. The median length of treatment for outpatients was 60 days (IQR 38-92) compared with 19 days (IQR 10-31) for inpatients (P < .001). What was interesting as well is that 82% of babies were discharged home on phenobarbital and 9.1% on methadone and 7.4% with both. A very small minority was discharged home on something else such as morphine or clonidine. That there was a tripling of medication wean is not surprising as once the patients are out of the watchful eye of the medical team in hospital it is likely that practitioners would use a very slow wean out of hospital to minimize the risk of withdrawal.
An Unintended Consequence
This study found a statistically significant increase in risk for presenting to the emergency department for those patients treated as outpatients.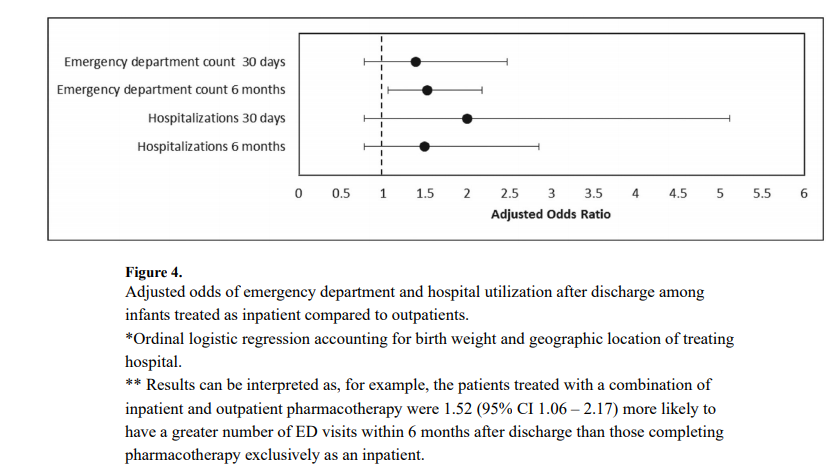

What this graph demonstrates is that there was no increase risk in the first month but there was for the first 6 months. Despite the increased risk of presentation to the ED the rate of hospitalization was not different. Drilling down the data further, the reason for coming to the ED was not for withdrawal which was 10% in the outpatient and 11% in the inpatient group. The other major reason was The most common diagnoses were upper respiratory infections; 80% outpatient vs 71% inpatient. So while there was a significant difference (which was not by much) my take on it is that it was most likely by chance as I can’t think of how infections in the first 6 months could be linked to choice of medication wean.
What about phenobarbital?
Phenobarbital has been used for many years in Neonatology for control of seizures, sedation (taking advantage of a side effect) and management of NAS. The problem with a median use of phenobarbital for 2 months is its potential to affect development.

An animal study by Diaz in 1999 in which rat pups were given two weeks of phenobarbital starting on day 5 of life and then euthanized demonstrated the following weight reductions when high dose phenobarbital was utilized. In human data, children with febrile seizures treated with phenobarbital in the paper Late cognitive effects of early treatment with phenobarbital. had decreased intelligence than those not exposed to phenobarbital.
The issue here for me is not necessarily whether babies can be treated successfully as outpatients for NAS. The concern is at what cost if the choice of drug is phenobarbital. The reason phenobarbital was chosen is likely due to compliance. We know that the more frequently a drug is dose the less likely compliance will be achieved. Phenobarbital being dosed either q12h or q24h is an ideal drug from a compliance point of view but the ramifications of this treatment deserve reconsideration.
I look forward to seeing further studies on this topic and hope that we see the results of an opioid outpatient treatment program. I know these exist and would welcome any information you as the readers of this blog can offer. Treating patients in the home makes great sense to me but we need to do it with the right drugs!

by All Things Neonatal | Jan 11, 2018 | NAS, Neonatal, neonatal abstinence, Neonatology, preemie
I wish it were otherwise, but in my practice, I have seen a growing number of pregnancies complicated by signs of substance withdrawal in newborn babies. Print, online, and broadcast news sources include regular reports on the “opioid crisis”. Data from the Canadian Institute for Health Information indicate that in 2016-17, about 1 in 200 newborns in Canada were affected by symptoms of drug withdrawal after birth. As this represents an average, there are no doubt some centres with much higher rates, while others may seem far lower depending on local usage patterns. Wherever you practice, if you care for newborns, you must learn how to treat this.
If you ask a physician in training how best to treat such conditions, their first response is often to use a medication such as morphine, thinking that it is best to treat an opioid withdrawal with the same class of drug. While this may be true, it is important to note that beginning with something much simpler, if not more natural, may reap tremendous benefits.
The Canadian Pediatric Society (CPS) released a new practice point this week, Managing infants born to mothers who have used opioids during pregnancy. While the document addresses the use of medical treatment, it highlights something far more important. Think of managing such pregnancies as a pyramid, with substance avoidance (the best strategy) on the bottom. The next level would be to manage newborns by keeping mothers and babies together. The top of the pyramid—that is, the fewest number of cases—would be treating these babies with medications.
For many families, avoidance is just not possible. Whether mothers use opioids due to addiction or chronic pain, it is simply unsafe to quit cold turkey. In October 2017, the Society of Obstetricians and Gynaecologists (SOGC) recommended against opioid detoxification in pregnancy because of the high risk of relapse. We should commend pregnant women who take responsibility for their health and seek care to stabilize on medications such as methadone or buprenorphine to manage their symptoms. After delivery, though, taking these babies and placing them on medications in a special care nursery should be a last resort.
Getting back to nature
Medications do work, but giving them means admitting babies to special care nurseries. This forced separation from families and, in particular, their mothers, actually leads to longer stays in hospital. Skin-to-skin care and breastfeeding contribute to better bonding between mother and child and have been associated with shortened hospital stays. In our centre, we have seen great success with many infants managed for up to seven days on the post-partum ward with their families. While this may seem like a long time, it is less than half of the average 15-day stay when babies are admitted to a special care unit.
Provided a mother is HIV-negative, the benefits of breastfeeding may go well beyond the bonding and closeness associated between mother and newborn. As most of these women continue to use a substance to ease their own withdrawal or pain, the small quantities of opioid that enter the breastmilk are in turn passed on to the newborn, which helps ease them through this transitional period in their life.
As the saying goes, sometimes less is more. In the case of caring for newborns exposed to opioids in pregnancy, getting back to nature and promoting skin-to-skin care and breastfeeding is just what this doctor ordered.
by All Things Neonatal | Nov 8, 2017 | Neonatal, neonatal abstinence, Neonatology
It would seem that the Opioid crisis is continuing to be front and centre in the news. Just today the President of the United States declared an Opioid Epidemic Emergency. Of course he was speaking primarily about the damage these drugs do on the family unit and those around them, the impact on the unborn child is significant as well. If this sounds familiar it is because I have written about this topic recently and in the past in the posts A Magic Bullet to Reduce Duration of Treatment and Hospital Stays for Newborns With NAS and Mandatory Drug-Testing ni PRegnancy: Lesson learned. I suppose I write about this topic often as at least where I work this is a problem which just won’t go away and takes up a tremendous amount of resources.
What Can a Large Data Set Tell us?
Pediatrix medical group that you may well be familiar with has a lot of data that can be mined from the hospitals in their network. When it comes to buprenorphine there is a lot of data to look at. In this case the question posed by VN Tolia et al in thier paper Antenatal methadone vs buprenorphine exposure and length of hospital stay in infants admitted to the intensive care unit with neonatal abstinence syndrome was whether there is a difference in infants born to mothers who have been exposed to methadone vs burprenorphine. Specifically they chose to use length of stay as the primary outcome in a retrospective review of 3364 infants admitted for management of NAS. Of these infants, 2202 (65%) were exposed to methadone and 1162 (34%) to buprenorphine. Before we get into what the results actually were it is important to highlight what this study will not tell us. By looking only at admissions for NAS we do not know whether the use of buprenorphine in mothers actually reduced admission for NAS so we are only speaking of the babies who were afflicted with NAS.
When looking at the two groups, the median length of stay was 24 days for the methadone group and 21 for the buprenorphine which was found to be significantly different. In the secondary analysis another interesting finding (at least to me) was noted.
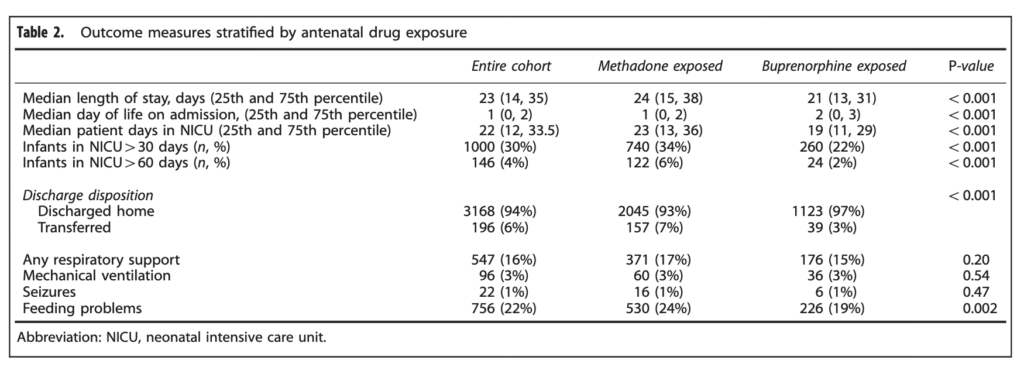 When looking at the percentage of babies with a length of stay > 30 days the difference was significant at 34% vs 22% for buprenorphine. The authors here did a good job of doing their best to control for factors which could have influenced the results as they did a regression analysis to determine whether other factors such as gestational age, sex, type of treatment provided etc would explain the shortened length of stay and they found that it remained significant controlling for a wide variety of factors.
When looking at the percentage of babies with a length of stay > 30 days the difference was significant at 34% vs 22% for buprenorphine. The authors here did a good job of doing their best to control for factors which could have influenced the results as they did a regression analysis to determine whether other factors such as gestational age, sex, type of treatment provided etc would explain the shortened length of stay and they found that it remained significant controlling for a wide variety of factors.
Is three days worth it?
It would be tempting to look at the 3 day median difference and shrug it off as no big deal. Remember though that we are in an epidemic are we not? What the study does not account for as well are the number of babies who could have been managed in a postpartum ward and also had a shortened length of stay. Let’s look at a city though where about 100 babies are admitted a year with NAS. A three day reduction in length of stay would translate into 300 patient days per year. By simply changing the medication a woman is being treated with in pregnancy from methadone to buprenorphine we could save almost one NICU bed for the whole year. That is nothing to sneeze at! Moreover if the reduction in admission rates are also true another one, two or more beds per year could be spared depending on the effectiveness of the drug.
In the last post that spoke of using buprenorphine to treat NAS in babies I was concerned about the alcohol content of the syrup for administration in babies. Here we are talking about treating women rather than babies so this is not a concern (plus they would not be taking the pediatric suspension). I see little downside to using buprenorphine over methadone so the real question is how do we get the care providers for the mothers to make the switch? I have a feeling that is coming sooner rather than later.

by All Things Neonatal | Sep 22, 2017 | neonatal abstinence, Neonatology, newborn
As someone with an interest in neonatal abstinence (NAS) I am surprised that I missed this study back in May. Anyone who says they aren’t interested in NAS research must be turning a blind eye to the North American epidemic of patients filling neonatal units or postpartum wards in need of treatment for the same. News feeds such as CNN have covered this story many times with concerning articles such as this published “Opioid Crisis Fast Facts” even the Trump White House has officially declared it as an emergency at this point. With NICU resources stretched and care providers fatigue levels wearing thin (these patients are typically very challenging to take care of due to the crying and agitation with neurological excitability that is at the core of the symptoms, something needs to be done. The vast majority of neonatal care providers treat such patients with an approach that promotes first non pharmacologic strategies such as keeping mom and baby together when possible, breast feeding and disturbing these infants as little as possible to name a few points. For those patients though who require pharmacologic support though, the mainstay has been oral morphine. At least in our units though once a patient is admitted and undergoes treatment we are still looking at anywhere from 3-4 weeks on average that they will occupy a hospital bed. If only there was a better way.
Could Buprenorphine do the trick?
While morphine is widely used to treat NAS symptoms unresponsive to other non pharmacologic methods of control, buprenorphine has a similar profile as an opioid but has less risk of respiratory depression as a partial agonist. A small but important trial has been published directly comparing the use of morphine to buprenorphine for treatment of NAS symptoms with the primary outcome being days of treatment and the second important point being length of stay. The trial, Buprenorphine for the Treatment of the Neonatal Abstinence Syndrome.by Kraft WK et al was entitled the BBORN trial for short. This was a single centre trial in which a double blind/double dummy approach was used. By double dummy this meant that after randomization those babies randomized to morphine received morphine plus a buprenorphine placebo and the other arm received a buprenorphine dose and a morphine placebo. In total 33 infants were randomized to buprenorphine and 30 to morphine (hence my comment about this being a small study). Their power calculation had called for 40 infants per arm to detect a 28% difference in the primary end point of duration of treatment but in the end that didn’t matter so much as they found a significant difference exceeding their estimate anyway. A lack of power would have become important mind you had they not found a difference as they wouldn’t have actually had the numbers to do so.
A strength of the study up front was that all care providers scored NAS symptoms the same way (need to take into account there is some subjectivity in scoring altogether though) and escalations and decreases of medication were done following a strict protocol both ways. In both arms, once a maximal dose of 60 mcg/kg of body weight for buprenorphine and 1.2 mg/kg for morphine was reached phenobarbital was added. When comparing the two groups at the outset there were no significant differences in characteristics so two generally similar populations of infants were being treated.
The Results Were Indeed Impressive
Before launching into the table, there were 21 babies in both groups that were bottle fed and 12 in the burprenorphine group and 9 in the morphine group that breastfed.
| Outcome |
Buprenorphine |
Morphine |
p |
| Median days of treatment |
15 (3-67) |
28 (13-67) |
<0.001 |
| Bottle feeding |
15 (3-67) |
28 (13-67) |
|
| Breast feeding |
20 (3-55) |
28 (16-52) |
|
|
|
|
|
| Hospital stay in days |
21 (7-71) |
33 (18-70) |
<0.001 |
| Bottle feeding |
21 (7-71) |
33 (18-70) |
|
| Breast feeding |
26 (7-58) |
32 (20-58) |
|
No difference was seen in those who needed phenobarbital. Looking at the table, a couple things really stand out to me. They were looking for a 28% reduction in days of treatment. The results came in far excess of that at a 46% reduction. Curiously, breastfeeding which has classically been associated with a reduction in scores and therefore faster weaning due to less symptoms seemed to have the opposite effect here. Does this imply that breastfeeding slows down both duration of treatment and length of stay as a result? With a study this small it is difficult to say with so few breastfed babies but if I had to guess I would suggest those mothers that worked at breastfeeding may have had longer stays.
Should we all jump on the buprenorphine train?
For now I would give this a big maybe. One of the concerns about burprenorphine is that it comes as a solution of 30% alcohol. Giving multiple doses (3 per day in this study) of such a solution could in part contribute to these results of lower NAS symptoms. Is giving alcohol to reduce symptoms a good idea here? Not sure if there are any long term effects and moreover if the cumulative dose of this medication would be of a concern. Definitely something to check with your local pharmacist before rolling this out. On the other hand if the dose of alcohol provided was truly significant I might have expected the burprenorphine group to be poorer feeders due to intoxication which we certainly did not see.
With increasing volumes of newborns afflicted with symptoms of NAS we do need to find a way to stem the tide. Ideally, primary preventative strategies would be best but until that solution is found could burprenorphine be the next step in tackling this epidemic?

by All Things Neonatal | Jun 8, 2017 | Neonatal, neonatal abstinence, Neonatology
I don’t envy our nurses who care for babies withdrawing from various opiates and other substances. These assignments are definitely a challenge and require a great deal of patience and depending on the shrillness of an infant’s cry a good set of earplugs. Nonetheless we do our best with these infants to keep them calm and avoid as much stimulation as we can as we attempt to minimize the excitability of their nervous system.
Over 40 years ago the Finnegan Neonatal Abstinence scoring system was developed to assist medical teams by providing as objective a system as possible to compare one infant to another and determine when and if a patient should be treated pharmacologically. Unfortunately there is a problem inherent with this scoring system. It is the same problem that exists when you don’t have a blinded research trial. Imagine you are caring for an infant and you were given no history about drug exposure. How might you score a patient like that compared to one in which you are told has been exposed to illicit substances? Your senses are heightened and moreover if you were told this baby is “withdrawing terribly” or “is awful at night” you are biased. How are you likely to score such a patient when they are “on the edge” of being counted as a 1 or a 0 in a category? I bet in many cases, especially if you haven’t taken care of many such patients you err on the side of caution and score them on the high side. It is human nature. When the possible outcome of failing to recognize a withdrawing patient is a seizure, no one wants to be on when it happens having their scoring questioned. Have a look at the scoring tool though.
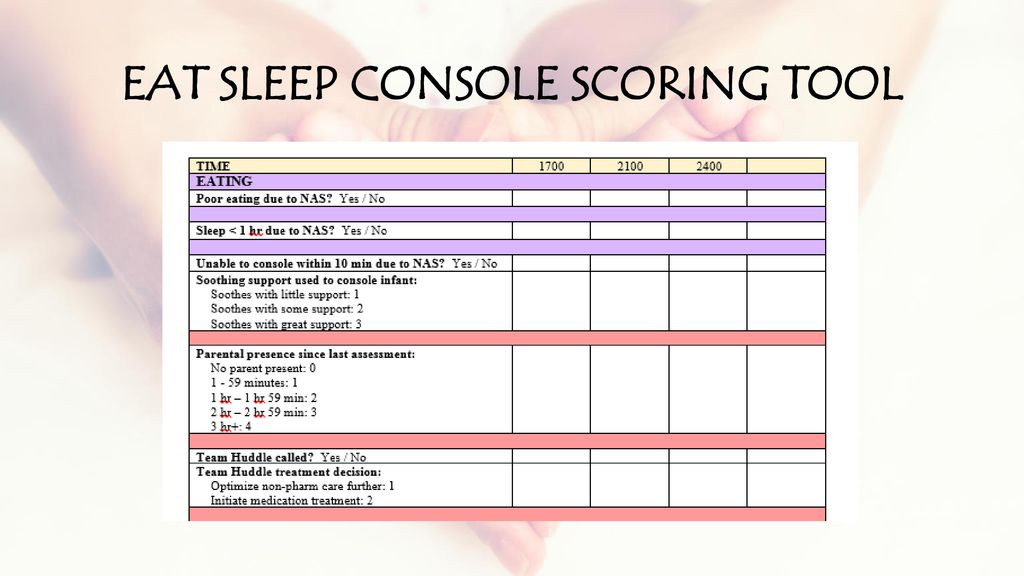
Neonatal Abstinence Syndrome – Eat Sleep Console Score Mode. EAT SLEEP CONSOLE SCORING TOOL.
There is a lot of stuff in there to check off! What if it could be simpler?
The ESC Tool
In early May, news began to break of an abstract being presented at the Pediatric Academic Society meeting. The news story from the AAP can be found here. The ESC tool is a three question tool used to assess whether an infant requires treatment for withdrawal.
E – Eat (is an infant is able to eat 1 or more ounce per feeding)
S – Sleeping (sleep for an hour or longer undisturbed)
C – Console (Be consoled in 10 minutes or less.)
If all three criteria are met, the patient does not need treatment. If one or more criteria are not met the housestaff are notified and first non-pharmacologic and then pharmacologic means are employed if necessary to control symptoms.
The authors did something quite interesting. They looked at 50 patients with 201 hospital days with prenatal exposures to opiates and applied the ESC criteria to guide treatment. Concurrently they captured the Finnegan scores but did not use them to guide treatment.
The findings I hope you will agree are quite interesting!
“FNASS scores indicated starting morphine in 30 infants (60%). Morphine was actually started on only 6 patients (12%) (p< 0.0001) based on the ESC approach. The FNASS led protocol directed initiating or increasing meds on 24.6% of days compared to 2.7% of days using the ESC approach (p< 0.0001). The FNASS approach directed that morphine was either not started or decreased on 65.8% of days compared with 94.4% of days using the ESC approach (p< 0.0001). There were no readmissions or reported adverse events.
Pretty amazing but…
The ESC approach greatly reduced the need for treatment and as the authors state there were no readmissions or reported adverse events. What we don’t know and will be needed I suspect before anyone will adopt this strategy (which I have to say again is so much simpler that current approaches) is how these children do in the long run. If the system is undertreating withdrawal, could we see downstream impacts of a “kinder and gentler” approach? One outcome that will be reported soon in the next month is length of stay. I am eagerly awaiting further results as I for one think that a simpler approach to these patients may be just what the doctor ordered. I think the nurses might thank us as well but we will see just how appropriate it is!
The Abstract reporting these findings can be found below
Novel Approach to Evaluating and Treating Infants with Neonatal Abstinence Syndrome

 This post is very timely as the CPS Fetus and Newborn committee has just released a new practice point:
This post is very timely as the CPS Fetus and Newborn committee has just released a new practice point:





 When looking at the percentage of babies with a length of stay > 30 days the difference was significant at 34% vs 22% for buprenorphine. The authors here did a good job of doing their best to control for factors which could have influenced the results as they did a regression analysis to determine whether other factors such as gestational age, sex, type of treatment provided etc would explain the shortened length of stay and they found that it remained significant controlling for a wide variety of factors.
When looking at the percentage of babies with a length of stay > 30 days the difference was significant at 34% vs 22% for buprenorphine. The authors here did a good job of doing their best to control for factors which could have influenced the results as they did a regression analysis to determine whether other factors such as gestational age, sex, type of treatment provided etc would explain the shortened length of stay and they found that it remained significant controlling for a wide variety of factors.

