by All Things Neonatal | Nov 8, 2017 | Neonatal, neonatal abstinence, Neonatology
It would seem that the Opioid crisis is continuing to be front and centre in the news. Just today the President of the United States declared an Opioid Epidemic Emergency. Of course he was speaking primarily about the damage these drugs do on the family unit and those around them, the impact on the unborn child is significant as well. If this sounds familiar it is because I have written about this topic recently and in the past in the posts A Magic Bullet to Reduce Duration of Treatment and Hospital Stays for Newborns With NAS and Mandatory Drug-Testing ni PRegnancy: Lesson learned. I suppose I write about this topic often as at least where I work this is a problem which just won’t go away and takes up a tremendous amount of resources.
What Can a Large Data Set Tell us?
Pediatrix medical group that you may well be familiar with has a lot of data that can be mined from the hospitals in their network. When it comes to buprenorphine there is a lot of data to look at. In this case the question posed by VN Tolia et al in thier paper Antenatal methadone vs buprenorphine exposure and length of hospital stay in infants admitted to the intensive care unit with neonatal abstinence syndrome was whether there is a difference in infants born to mothers who have been exposed to methadone vs burprenorphine. Specifically they chose to use length of stay as the primary outcome in a retrospective review of 3364 infants admitted for management of NAS. Of these infants, 2202 (65%) were exposed to methadone and 1162 (34%) to buprenorphine. Before we get into what the results actually were it is important to highlight what this study will not tell us. By looking only at admissions for NAS we do not know whether the use of buprenorphine in mothers actually reduced admission for NAS so we are only speaking of the babies who were afflicted with NAS.
When looking at the two groups, the median length of stay was 24 days for the methadone group and 21 for the buprenorphine which was found to be significantly different. In the secondary analysis another interesting finding (at least to me) was noted.
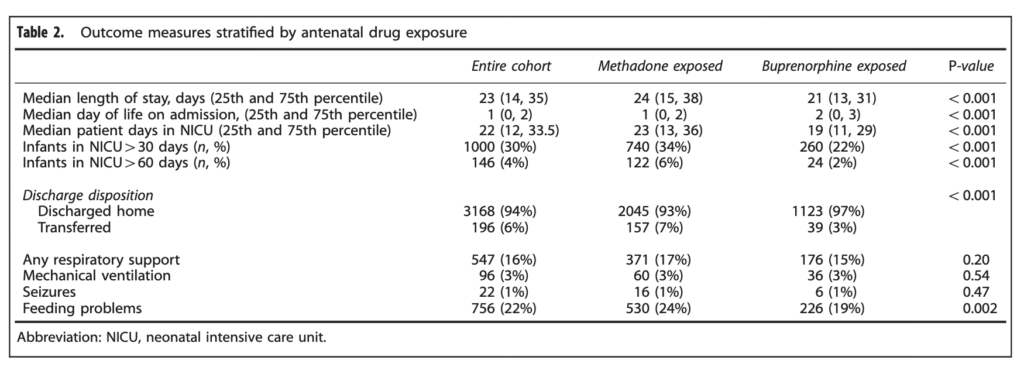 When looking at the percentage of babies with a length of stay > 30 days the difference was significant at 34% vs 22% for buprenorphine. The authors here did a good job of doing their best to control for factors which could have influenced the results as they did a regression analysis to determine whether other factors such as gestational age, sex, type of treatment provided etc would explain the shortened length of stay and they found that it remained significant controlling for a wide variety of factors.
When looking at the percentage of babies with a length of stay > 30 days the difference was significant at 34% vs 22% for buprenorphine. The authors here did a good job of doing their best to control for factors which could have influenced the results as they did a regression analysis to determine whether other factors such as gestational age, sex, type of treatment provided etc would explain the shortened length of stay and they found that it remained significant controlling for a wide variety of factors.
Is three days worth it?
It would be tempting to look at the 3 day median difference and shrug it off as no big deal. Remember though that we are in an epidemic are we not? What the study does not account for as well are the number of babies who could have been managed in a postpartum ward and also had a shortened length of stay. Let’s look at a city though where about 100 babies are admitted a year with NAS. A three day reduction in length of stay would translate into 300 patient days per year. By simply changing the medication a woman is being treated with in pregnancy from methadone to buprenorphine we could save almost one NICU bed for the whole year. That is nothing to sneeze at! Moreover if the reduction in admission rates are also true another one, two or more beds per year could be spared depending on the effectiveness of the drug.
In the last post that spoke of using buprenorphine to treat NAS in babies I was concerned about the alcohol content of the syrup for administration in babies. Here we are talking about treating women rather than babies so this is not a concern (plus they would not be taking the pediatric suspension). I see little downside to using buprenorphine over methadone so the real question is how do we get the care providers for the mothers to make the switch? I have a feeling that is coming sooner rather than later.
by All Things Neonatal | Oct 25, 2017 | Neonatal, preemie, Prematurity, ventilation
A patient has been extubated to CPAP and is failing with increasing oxygen requirements or increasing apnea and bradycardia. In most cases an infant would be reintubated but is there another way? While CPAP has been around for some time to support our infants after extubation, a new method using high frequency nasal ventilation has arrived and just doesn’t want to go away. Depending on your viewpoint, maybe it should or maybe it is worth a closer look. I have written about the modality before in High Frequency Nasal Ventilation: What Are We Waiting For? While it remains a promising technology questions still remain as to whether it actually delivers as promised.
Better CO2 elimination?
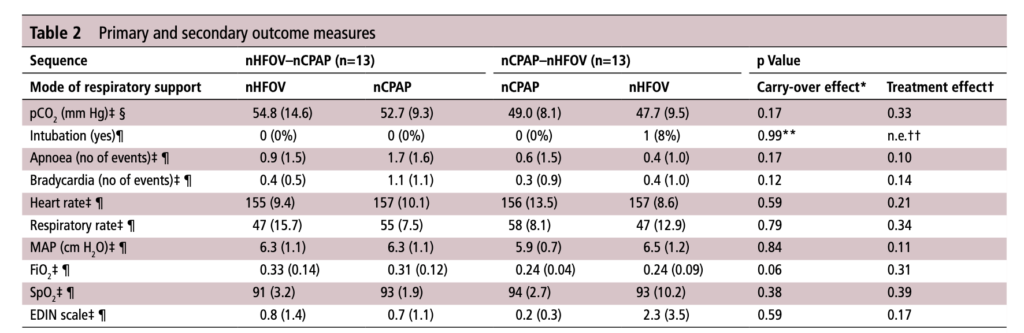
For those who have used a high frequency oscillator, you would know that it does a marvelous job of removing CO2 from the lungs. If it does so well when using an endotracheal tube, why wouldn’t it do just as good a job when used in a non-invasive way? That is the hypothesis that a group of German Neonatologists put forth in their paper this month entitled Non-invasive high-frequency oscillatory ventilation in preterm infants: a randomised controlled crossover trial. In this relatively small study of 26 preterm infants who were all less than 32 weeks at delivery, babies following extubation or less invasive surfactant application were randomized to either receive nHFOV then CPAP for four hours each or the reverse order for the same duration. The primary outcome here was reduction in pCO2 with the goal of seeking a difference of 5% or more in favour of nHFOV. Based on their power calculation they thought they would need 24 infants total and therefore exceeded that number in their enrollment.
The babies in both arms were a bit different which may have confounded the results. The group randomized to CPAP first were larger (mean BW 1083 vs 814g), and there was a much greater proportion of males in the CPAP group. As well, the group randomized first to CPAP had higher baseline O2 saturation of 95% compared to 92% in the nHFOV group. Lastly and perhaps most importantly, there was a much higher rate of capillary blood sampling instead of arterial in the CPAP first group (38% vs 15%). In all cases the numbers are small but when looking for such a small difference in pCO2 and the above mentioned factors tipping the scales one way or the other in terms of illness severity and accuracy of measurement it does give one reason to pause when looking at the results.
The Results
No difference was found in the mean pCO2 from the two groups. As expected, pCO2 obtained from capillary blood gases nearly met significance for being higher than arterial samples (50 vs 47; p=0.052). A similar rate of babies had to drop out of the study (3 on the nCPAP first and 2 on the nHFOV side).
In the end should we really be surprised by the results? I do believe that in the right baby who is about to fail nCPAP a trial of nHFOV may indeed work. By what means I really don’t understand. Is it the fact that the mean airway pressure is generally set higher than on nCPAP in some studies? Could it be the oscillatory vibration being a kind of noxious stimulus that prevents apneic events through irritation of the infant?
While traditional invasive HFOV does a marvelous job of clearing out CO2 I have to wonder how the presence of secretions and a nasopharynx that the oscillatory wave has to avoid (almost like a magic wave that takes a 90 degree turn and then moves down the airway) allows much of any of the wave to reach the distal alveoli. It would be similar to what we know of inhaled steroids being deposited 90 or so percent in the oral cavity and pharynx. There is just a lot of “stuff” in the way from the nostril to the alveolus.
This leads me to my conclusion that if it is pCO2 you are trying to lower, I wouldn’t expect any miracles with nHFOV. Is it totally useless? I don’t think so but for now as a respiratory modality I think for the time being it will continue to be “looking for a place to happen”
by All Things Neonatal | Oct 19, 2017 | BPD, Neonatal, Neonatology, preemie, Prematurity
If you work in Neonatology then chances are you have ordered or assisted with obtaining many chest x-rays in your time. If you look at home many chest x-rays some of our patients get, especially the ones who are with us the longest it can be in the hundreds. I am happy to say the tide though is changing as we move more and more to using other imaging modalities such as ultrasound to replace some instances in which we would have ordered a chest x-ray. This has been covered before on this site a few times; see Point of Care Ultrasound in the NICU, Reducing Radiation Exposure in Neonates: Replacing Radiographs With Bedside Ultrasound. and Point of Care Ultrasound: Changing Practice For The Better in NICU.This post though is about something altogether different.
If you do a test then know what you will do with the result before you order it.
If there is one thing I tend to harp on with students it is to think about every test you do before you order it. If the result is positive how will this help you and if negative what does it tell you as well. In essence the question is how will this change your current management. If you really can’t think of a good answer to that question then perhaps you should spare the infant the poke or radiation exposure depending on what is being investigated. When it comes to the baby born before 30 weeks these infants are the ones with the highest risk of developing chronic lung disease. So many x-rays are done through their course in hospital but usually in response to an event such as an increase in oxygen requirements or a new tube with a position that needs to be identified. This is all reactionary but what if you could do one x-ray and take action based on the result in a prospective fashion?
What an x-ray at 7 days may tell you
How many times have you caught yourself looking at an x-ray and saying out loud “looks like evolving chronic lung disease”. It turns out that Kim et al in their publication Interstitial pneumonia pattern on day 7 chest radiograph predicts bronchopulmonary dysplasia in preterm infants.believe that we can maybe do something proactively with such information.
In this study they looked retrospectively at 336 preterm infants weighing less than 1500g and less than 32 weeks at birth. Armed with the knowledge that many infants who have an early abnormal x-ray early in life who go on to develop BPD, this group decided to test the hypothesis that an x-ray demonstrating a pneumonia like pattern at day 7 of life predicts development of BPD. 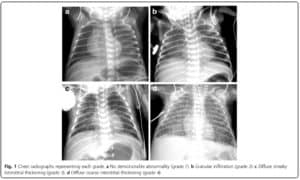 The patterns they were looking at are demonstrated in this figure from the paper. Essentially what the authors noted was that having the worst pattern of the lot predicted the development of later BPD. The odds ratio was 4.0 with a confidence interval of 1.1 – 14.4 for this marker of BPD. Moreover, birthweight below 1000g, gestational age < 28 weeks and need for invasive ventilation at 7 days were also linked to the development of the interstitial pneumonia pattern.
The patterns they were looking at are demonstrated in this figure from the paper. Essentially what the authors noted was that having the worst pattern of the lot predicted the development of later BPD. The odds ratio was 4.0 with a confidence interval of 1.1 – 14.4 for this marker of BPD. Moreover, birthweight below 1000g, gestational age < 28 weeks and need for invasive ventilation at 7 days were also linked to the development of the interstitial pneumonia pattern.
What do we do with such information?
I suppose the paper tells us something that we have really already known for awhile. Bad lungs early on predict bad lungs at a later date and in particular at 36 weeks giving a diagnosis of BPD. What this study adds if anything is that one can tell quite early whether they are destined to develop this condition or not. The issue then is what to do with such information. The authors suggest that by knowing the x-ray findings this early we can do something about it to perhaps modify the course. What exactly is that though? I guess it is possible that we can use steroids postnatally in this cohort and target such infants as this. I am not sure how far ahead this would get us though as if I had to guess I would say that these are the same infants that more often than not are current recipients of dexamethasone.
Would another dose of surfactant help? The evidence for late surfactant isn’t so hot itself so that isn’t likely to offer much in the way of benefit either.
In the end the truth is I am not sure if knowing concretely that a patient will develop BPD really offers much in the way of options to modify the outcome at this point. Having said that the future may well bring the use of stem cells for the treatment of BPD and that is where I think such information might truly be helpful. Perhaps a screening x-ray at 7 days might help us choose in the future which babies should receive stem cell therapy (should it be proven to work) and which should not. I am proud to say I had a chance to work with a pioneer in this field of research who may one day cure BPD. Dr. Thebaud has written many papers of the subject and if you are looking for recent review here is one Stem cell biology and regenerative medicine for neonatal lung diseases.Do I think that this one paper is going to help us eradicate BPD? I do not but one day this strategy in combination with work such as Dr. Thebaud is doing may lead us to talk about BPD at some point using phrases like “remember when we used to see bad BPD”. One can only hope.
by All Things Neonatal | Sep 13, 2017 | Neonatal, Neonatology, newborn, preemie, Prematurity, resuscitation, ventilation
We can always learn and we can always do better. At least that is something that I believe in. In our approach to resuscitating newborns one simple rule is clear. Fluid must be replaced by air after birth and the way to oxygenate and remove CO2 is to establish a functional residual capacity. The functional residual capacity is the volume of air left in the lung after a tidal volume of air is expelled in a spontaneously breathing infant and is shown in the figure. Traditionally, to establish this volume in a newborn who is apneic, you begin PPV or in the spontaneously breathing baby with respiratory distress provide CPAP to help inflate the lungs and establish FRC.
Is there another way?
Something that has been discussed now for some time and was commented on in the most recent version of NRP was the concept of using sustained inflation (SI) to achieve FRC. I have written about this topic previously and came to a conclusion that it wasn’t quite ready for prime time yet in the piece Is It Time To Use Sustained Lung Inflation In NRP?
The conclusion as well in the NRP textbook was the following:
“There are insufficient data regarding short and long-term safety and the most appropriate duration and pressure of inflation to support routine application of sustained inflation of greater than 5 seconds’ duration to the transitioning newborn (Class IIb, LOE B-R). Further studies using carefully designed protocols are needed”
So what now could be causing me to revisit this concept? I will be frank and admit that whenever I see research out of my old unit in Edmonton I feel compelled to read it and this time was no different. The Edmonton group continues to do wonderful work in the area of resuscitation and expand the body of literature in such areas as sustained inflation.
Can you predict how much of a sustained inflation is needed?
This is the crux of a recent study using end tidal CO2 measurement to determine whether the lung has indeed established an FRC or not. Dr. Schmolzer’s group in their paper (Using exhaled CO2 to guide initial respiratory support at birth: a randomised controlled trial) used end tidal CO2 levels above 20 mmHg to indicate that FRC had been established. If you have less CO2 being released the concept would be that the lung is actually not open. There are some important numbers in this study that need to be acknowledged. The first is the population that they looked at which were infants under 32 6/7 weeks and the second is the incidence of BPD (need for O2 or respiratory support at 36 weeks) which in their unit was 49%. This is a BIG number as in comparison for infants under 1500g our own local incidence is about 11%. If you were to add larger infants closer to 33 weeks our number would be lower due to dilution. With such a large number though in Edmonton it allowed them to shoot for a 40% reduction in BPD (50% down to 30%). To accomplish this they needed 93 infants in each group to show a difference this big.
So what did they do?
For this study they divided the groups in two when the infant wouldn’t breathe in the delivery room. The SI group received a PIP of 24 using a T-piece resuscitator for an initial 20 seconds. If the pCO2 as measured by the ETCO2 remained less than 20 they received an additional 10 seconds of SI. In the PPV group after 30 seconds of PPV the infants received an increase of PIP if pCO2 remained below 20 or a decrease in PIP if above 20. In both arms after this phase of the study NRP was then followed as per usual guidelines.
The results though just didn’t come through for the primary outcome although ventilation did show a difference.
| Outcome |
SI |
PPV |
p |
| BPD |
23% |
33% |
0.09 |
| Duration of mechanical ventilation (hrs) |
63 |
204 |
0.045 |
The reduction in hours of ventilation was impressive although no difference in BPD was seen. The problem though with all of this is what happened after recruitment into the study. Although they started with many more patients than they needed, by the end they had only 76 in the SI group and 86 in the PPV group. Why is this a problem? If you have less patients than you needed based on the power calculation then you actually didn’t have enough patients enrolled to show a difference. The additional compounding fact here is that of the Hawthorne Effect. Simply put, patients who are in a study tend to do better by being in a study. The observed rate of BPD was 33% during the study. If the observed rate is lower than expected when the power calculation was done it means that the number needed to show a difference was even larger than the amount they originally thought was needed. In the end they just didn’t have the numbers to show a difference so there isn’t much to conclude.
What I do like though
I have a feeling or a hunch that with a larger sample size there could be something here. Using end tidal pCO2 to determine if the lung is open is in and of itself I believe a strategy to consider whether giving PPV or one day SI. We already use colorimetric devices to determine ETT placement but using a quantitative measure to ascertain the extent of open lung seems promising to me. I for one look forward to the continued work of the Neonatal Resuscitation–Stabilization–Triage team (RST team) and congratulate them on the great work that they continue doing.
by All Things Neonatal | Aug 2, 2017 | Neonatal, Neonatology, newborn, Pain in the Neonate, preemie
I would consider myself fairly open minded when it comes to care in the NICU. I wouldn’t call myself a maverick or careless but I certainly am open to new techniques or technologies that may offer a better level of care for the babies in our unit. When it comes to “non-Western” concepts though such as therapeutic touch, chiropractic manipulations of infants and acupuncture (needle or otherwise) I have generally been a skeptic. I have written about such topics before with the most popular post being Laser acupuncture for neonatal abstinence syndrome. My conclusion there was that I was not a fan of the strategy but perhaps I could be more open to non traditional therapies.
Magnetic Acupuncture
This would appear to be the newest and perhaps strangest (to me at least) approach to pain relief that I have seen. I do love name of this study; the MAGNIFIC trial consisted of a pilot study on the use of auricular magnetic acupuncture to alleviate pain in the NICU from heel lances. The study was published in Acta Paediatrica this month; Magnetic Non-Invasive Acupuncture for Infant Comfort (MAGNIFIC) – A single-blinded randomized controlled pilot trial. The goal here was to measure pain scores using the PIPP scoring system for pain in the neonate before during and after a painful experience (heel lance) in the NICU. Being a pilot study it was small with only 20 needed per arm based on the power calculation to detect a 20% difference in scores. The intervention used small magnets placed at specific locations on the ear of the infant at least two hours before the heel lance was to occur. Before I get into the results, the authors of the study provide references to explain how the therapy works. Looking at the references I have to admit I was not able to obtain complete papers but the evidence is generally it would appear from adult patients. The explanation has to do with the magnetic field increasing blood flow to the area the magnet is applied to and in addition another reference suggests that there are affects the orbitofrontal and limbic regions which then impacts neurohormonal responses as seen in functional MRI. The evidence to support this is I would have thought would be pretty sparse but I was surprised to find a literature review on the subject that looked at 42 studies on the topic. The finding was that 88% of the studies reported a therapeutic effect. The conclusion though of the review was that the quality of the included studies was a bit sketchy for the most part so was not able to find that this should be a recommended therapy.
So what were the results?
Despite my clear skepticism what this study did well was that aside from the magnets, the intervention was the same. Twenty one babies received the magnetic treatments vs 19 placebo. There was a difference in the gestational ages of the babies with the magnet treated infants being about two weeks older (35 vs 33 weeks). What difference that might in and of itself have on the PIPPs scoring I am not sure. The stickers were applied to the ears with and without magnets in a randomized fashion and the nurses instructed to score them using the PIPP scoring system. Interestingly, as per their unit policy all babies received sucrose as well before the intervention of a heel lance so I suppose the information gleaned here would be the use of magnets as an adjunctive treatment. No difference was noted in the two groups before and after the heel lance but during the procedure the magnet treated infants had a difference in means (SD): 5.9 (3.7) v 8.3 (4.7), p=0.04). No differences were found in secondary measures such as HR or saturation and no adverse effects were noted. The authors conclusions were that it was feasible and appears safe and as with most pilot studies warrants further larger studies to verify the results.
Should we run out and buy it?
One of the issues I have with the study is that in the introduction they mention that this treatment might be useful where kangaroo care (KC) is not such as a critically ill infant. Having placed infants who are quite sick in KC and watched wonderful stability arise I am not sure if the unit in question under utilizes this important modality for comfort.
The second and perhaps biggest issue I have here is that although the primary outcome was reached it does seem that there was some fishing going on here. By that I mean there were three PIPP scores examined (before, during and after) and one barely reached statistical significance. My hunch is that indeed this was reached by chance rather than it being a real difference.
The last concern is that while the intervention was done in a blinded and randomized fashion, the evidence supporting the use of this in the first place is not strong. Taking this into account and adding the previous concern in as well and I have strong doubts that this is indeed “for real”. I doubt this will be the last we will hear about it and while my skepticism continues I have to admit if a larger study is produced I will be willing and interested to read it.

by All Things Neonatal | Jun 21, 2017 | ID, Infection, Neonatal, newborn
I had the pleasure of meeting the author of a paper I am about to comment on this week while at the 99 NICU conference in Stockholm. Dr. Ohlin from Orebro University in Sweden presented very interesting work on their unit’s “scrub the hub” campaign. As he pointed out, many places attempt to reduce coagulase negative staphylococcal infections by introducing central line bundles but seldom is there one thing that is changed in a bundle that allows for a before and after comparison like his team was able to do. I was so impressed by this work and at the same time concerned about another strategy to reduce infection that I felt compelled to make a comment here.
Scrub the hub!
Dr. Ohlin and the first author Dr. Bjorkman published Scrubbing the hub of intravenous catheters with an alcohol wipe for 15 sec reduced neonatal sepsis back in 2015. They compared a 16.5 month period in their unit when they rolled out a CLABI reduction bundle to a period of 8.5 months afterwards when they made one change. Nurses as is done in the units I work in were commonly scrubbing the hub before they injected the line with a medication but in the second epoch the standard changed to be a specified 15 second scrub instead of being left up to the individual nurse. With permission from Dr. Ohlin here is a picture of the hubs highlighting bacterial growth without scrubbing, then for a duration less than 15 seconds and then with 15 seconds.
In the first epoch they had 9 confirmed CLABSIs and 0 confirmed in the second after their intervention. The rate of CLABSI then in the first epoch was 1.5% vs 0% in the second group. As with any study looking at sepsis, definitions are important and while they didn’t do paired cultures to rule out contamination (one positive and one negative as is the definition in our hospitals) they did refer each patient to a senior Neonatologist to help determine whether each case should be considered a true positive or not. Given that they made no changes to practice or other definitions in diagnosing infections during that time perhaps the results were indeed real. Presumably if they had missed an infection and not treated it in the second epoch the patient would have declared themselves so I think it is reasonable to say that 8.5 months without a CLABSI after their intervention is a success. As Dr. Ohlin points out the scrub duration may also help due to the abrasion of the hub surface removing a bacterial film. Regardless of the reason, perhaps a 15 second scrub is a good idea for all?
The lazy person’s solution – the SwabCap
One way to get around human nature or people being distracted might be to cover each luer lock with a cap containing 70% isopropyl alcohol. In this way when you go to access the line there should be no bacteria or labour required to scrub anything since the entry of the line is bathed in alcohol already. This was the subject of a systematic review from the Netherlands entitled Antiseptic barrier cap effective in reducing central line-associated bloodstream infections: A systematic review and meta-analysis. The reviews ultimately examined 9 articles that met their inclusion criteria and found the following; use of the antiseptic barrier cap was effective in reducing CLABSIs (IRR = 0.59, 95% CI = 0.45–0.77, P < 0.001). Moreover, they concluded that this was an intervention worth adding to central-line maintenance bundles. Having said that, the studies were mostly adult and therefore the question of whether minute quantities of isopropyl alcohol might be injected with medications was not a concern when they made their conclusion.
What about using such caps in ELBW infants
Sauron et al in St. Justine Hospital in Montreal chose to look at these caps more carefully after they were implemented in their NICU. The reason for taking a look at them was due to several luer valves malfunctioning. The authors created an in-vitro model to answer this question by creating a closed system in which they could put a cap on the end of a line with a luer lock and then inject a flush, followed by a simulated medication (saline) and then a flush and collect the injected materials in a glass vial that was sealed to prevent evaporative loss of any isopropyl alcohol. They further estimated the safe amount of isopropyl alcohol from Pediatric studies would be 1% of the critical threshold of this alcohol and using a 500g infant’s volume of distribution came up with a threshold of 14 mmol/L. The study then compared using the SwabCap over two different valve leur lock systems they had in their units (SmartSite and CARESITE valves) vs. using the strategy of “scrub the hub”.
The results were quite concerning and are shown below.
| Circuit Type |
Temperature |
Sample 1 |
Sample 2 |
Sample 3 |
Mean |
| SwabCap on Smart Site Valve |
Room |
49.5 |
58.4 |
46.8 |
51.6 |
|
Incubator 35 degrees |
45.16 |
94.7 |
77.9 |
72.6 |
| SwabCap on CARESITE valve |
Room |
14.1 |
5.7 |
5.2 |
8.34 |
|
Incubator 35 degrees |
7.0 |
8.1 |
5.9 |
7.0 |
| Isopropyl alcohol pad on CARESITE Valve |
Room |
0 |
0 |
0 |
0 |
Certainly, the Smart Site valve allowed considerable amounts of isopropyl alcohol to enter the line but the CARESITE while better still allowed entry compared to the control arm which allowed none. Beyond the introduction of the alcohol into the system in all cases considerable clouding of the valves occurred with repeated capping of the system with new caps as was done with each med injection since each was single use. In lines that were not accessed contact with the cap was left for 96 hours as per recommendations from the manufacturer and these changes occurred as well.
Conclusion
While a reduction in CLABSI is something we all need to strive to obtain, it is better to take the more difficult path and “scrub the hub” and by that for 15 seconds which incidentally is the same recommended duration for hand hygiene in both of our units. Perhaps in larger term infant’s seepage of isopropyl alcohol into the lines would not be as concerning as their larger volume of distribution would lead to lower levels but I would ask the question “should any isopropyl alcohol be injected into any baby?”. I think not and perhaps by reading this post you will ask the same thing if your unit is using these caps.
- Thank you to Örebro University Hospital for their permission in using the photo for the post

 When looking at the percentage of babies with a length of stay > 30 days the difference was significant at 34% vs 22% for buprenorphine. The authors here did a good job of doing their best to control for factors which could have influenced the results as they did a regression analysis to determine whether other factors such as gestational age, sex, type of treatment provided etc would explain the shortened length of stay and they found that it remained significant controlling for a wide variety of factors.
When looking at the percentage of babies with a length of stay > 30 days the difference was significant at 34% vs 22% for buprenorphine. The authors here did a good job of doing their best to control for factors which could have influenced the results as they did a regression analysis to determine whether other factors such as gestational age, sex, type of treatment provided etc would explain the shortened length of stay and they found that it remained significant controlling for a wide variety of factors.



 The patterns they were looking at are demonstrated in this figure from the paper. Essentially what the authors noted was that having the worst pattern of the lot predicted the development of later BPD. The odds ratio was 4.0 with a confidence interval of 1.1 – 14.4 for this marker of BPD. Moreover, birthweight below 1000g, gestational age < 28 weeks and need for invasive ventilation at 7 days were also linked to the development of the interstitial pneumonia pattern.
The patterns they were looking at are demonstrated in this figure from the paper. Essentially what the authors noted was that having the worst pattern of the lot predicted the development of later BPD. The odds ratio was 4.0 with a confidence interval of 1.1 – 14.4 for this marker of BPD. Moreover, birthweight below 1000g, gestational age < 28 weeks and need for invasive ventilation at 7 days were also linked to the development of the interstitial pneumonia pattern.
