by All Things Neonatal | Mar 31, 2016 | ID, Infection, Neonatal, Uncategorized
I have been a huge advocate of RSV prophylaxis since my days as a Pediatric resident. When I started my residency we were not using Palivizumab (Synagis) and I recall admitting 10+ patients per day at times with bronchiolitis. With the use of passive immunization this rate dropped dramatically in Manitoba although rates in other areas of the country may have not seen such significant impacts. Manitoba may be somewhat different from many areas due to the communities in Nunavut being so impacted when RSV enters these areas and can infect many of the children due to crowded living conditions and inability to really isolate kids from one and other. The lack of benefit in other areas though, has no doubt led to controversy among practitioners who often wonder if giving 5 IM injections during the RSV season is indeed worth it. The real question has not necessarily been does it work but to whom should it be given so that you get the most benefit.
A Big Change in The Last Year
In 2015 the CPS published a revised statement entitled Preventing hospitalizations for respiratory syncytial virus infection. This statement has caused a great deal of controversy at least among those I have spoken with due to its significant departure from the previous recommendations. As per the statement:
- In preterm infants without CLD born before 30 + 0 weeks’ GA who are <6 months of age at the start of RSV season, it is reasonable (but not essential) to offer palivizumab. Infants born after 30 + 0 weeks’ GA have RSV admission rates that are consistently ≤7% (Figure 3), yielding a minimum number needed to treat of 18 (90 doses of palivizumab to prevent one RSV admission) if one assumes 80% efficacy and five doses per infant. Therefore, palivizumab should not be prescribed for this group.
Gone are recommendations for treating those from 30 – 32 weeks and moreover 33- 35 weeks if meeting certain conditions. There is a provision for those in Northern communities to expand these criteria to 36 0/7 weeks if such infants would require medical transport to receive care for bronchiolitis. What is not really clear though is what is meant by Northern communities in terms of criteria to determine suitability exactly. Incidentally, the criteria are not so different than the AAP statement from August 2014.
Do We Need So Many Shots?
Just at the end of 2016 though Lavoie P et al in Vancouver, BC published a letter outlining their experience with a modified schedule of either 3 or 4 doses of palivizumab during the RSV season. Included in the letter are their criteria for determining the number of doses and importantly pharmacokinetic data demonstrating the effectiveness of such schedules in achieving protective titres. 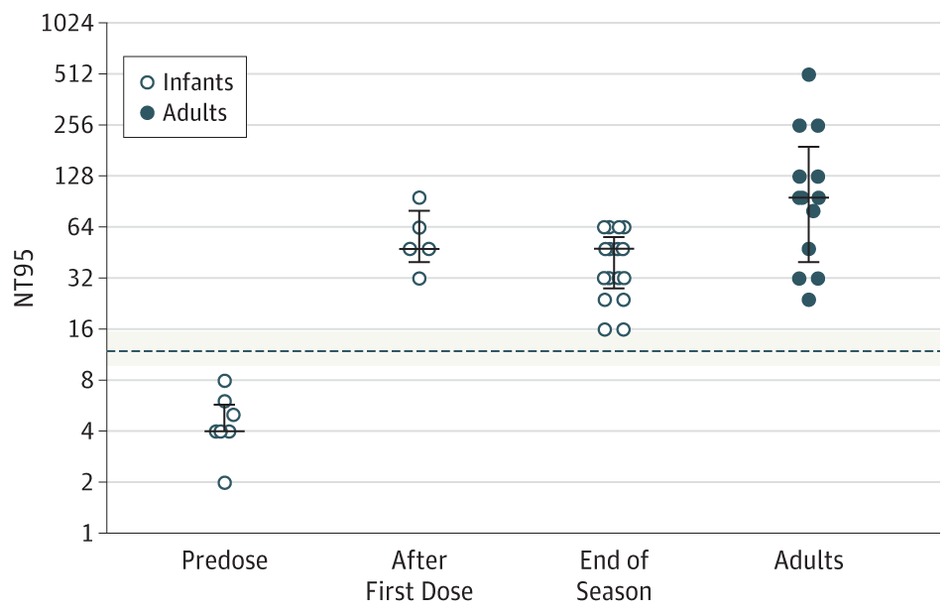 The 3 dose schedule was used for those infants born between 29 0/7 and 35 weeks gestational age who had a risk factor score of 42 or more. Interestingly at the end of the RSV season, depriving such infants of 1 or 2 doses did not appear to impair the ability of the infant to maintain protective levels.
The 3 dose schedule was used for those infants born between 29 0/7 and 35 weeks gestational age who had a risk factor score of 42 or more. Interestingly at the end of the RSV season, depriving such infants of 1 or 2 doses did not appear to impair the ability of the infant to maintain protective levels.
From a clinical standpoint the outcome data during this period examining 514 (3 dose) and 666 (4 dose) patients similarly suggests that they were indeed protected from disease. In the 3 dose cohort only 1 patient was hospitalized with RSV during the dosing period and 1 infant afterwards. In the 4 dose group, 10 were hospitalized with RSV during the dosing schedule and a set of twins afterwards. Aside from these known RSV infections, an additional 7 and 18 patients were hospitalized with bronchiolitis without viral identification during the dosing schedule with no cases of bronchiolitis afterwards. Taken altogether and assuming that all cases were indeed RSV bronchiolitis the authors conclude that the overall rates are no different than those seen with a 5 dose schedule.
Is Something Rotten In The State of Denmark?
There is something peculiar here though. There is no doubt that palivizumab must have gone through rigorous pharmacokinetic testing in order to determine the correct number of doses needed. For a 3-4 dose regimen to provide the same coverage in terms of antibody titres seems strange to me. I would love to believe the data but there is a skeptic in me. Secondly with respect to counting hospital admissions is this exhaustive in terms of including all hospitalization a in BC or at only some sites? Clarity is needed before considering such changes to practice. Strangely it has been several months since this experience was published and there has been no discussion of it at least locally.* Something as dramatic as this should have sparked some discussion and the absence of such leaves me questioning what am I missing?
From the standpoint of reducing interventions and pain in the neonate I am intrigued by these findings. Parents as well would no doubt be happier with 3-4 IM injections over 5. The additional benefit is no doubt financial as this product while effective does carry a significant cost per dose. As you can see I have my doubts about the reproducibility of the results but it does at least offer some centres that have not been as enthusiastic about palivizumab something to consider. For some, the BC approach just might be the right thing.
- I indicate that there has been little discussion locally of the article discussed. There has indeed been discussion both here and in other Canadian provinces. What I meant by that comment is that among my colleagues in Neonatology and Infectious Diseases and housestaff I have had only one discussion.
by All Things Neonatal | Mar 26, 2016 | transplantation, Uncategorized
It seems fitting that I would be writing this on the Easter holiday with the symbolism of death followed by resurrection being the principal path for an organ to live on in another after departing their host. This topic came across my radar this week after I caught the following article on NBC news that I would encourage you to take a look at as it really captures the state of this practice in the US at the moment.
The Littlest Donors:Neonatal Organ Donation Offers Hope in Tragedy
This may surprise you but there really is nothing in the literature about this. There was a flurry of articles about neonatal transplantation in the late 1980s as debate raged about the ethics of using anencephalic newborns as donors but since then very little. I could find one article in pubmed which is a basic science article showing that mid trimester fetuses lung tissue can be used to regenerate cells in a recipient lung scaffold many hours after the stillbirth had occurred with the longest duration being 41 hours in this study. It would seem based on this study that the capacity for stillborn tissue to survive outside the deceased in theory then could provide a window for obtaining consent although how long such whole organs would last is uncertain.
The Big Questions?
How as a medical society we could manage this is a challenging one. Consent would only be possible after the recognition of fetal loss and who knows how long a window there would be from the time of death until the baby was delivered. Approaching families to ask about organ donation after they have just suffered a fetal loss is a difficult task to say the least. On the other hand the opportunity for some families to have some good come out of their tragedy as the NBC article points out may be therapeutic in some way.
Lastly, there is no question that there is a need for small organs. Having worked previously in a centre that offered cardiac transplantation I recall waiting for available hearts that never came or came too late. The challenge is that the thorax is only so big in a newborn and can’t accommodate a heart from a child or adult. Add to this that certain conditions preclude a baby or child from donating depending on the cause of death and having access to a larger pool of potential organs could indeed change the landscape of organ transplantation.
I should also stress that I am not talking about Planned Parenthood who has been accused of selling aborted fetuses tissue (having said that I can find little hard evidence for these claims out there). These are all babies who have been stillborn and not intentionally born to harvest organs.
I doubt this is the last you will hear of this but as the topic becomes more mainstream I can see ethicists and legal teams coming to the forefront to tackle this at each hospital and across many countries. It will be intriguing to follow this story and see how it unfolds.
by All Things Neonatal | Mar 23, 2016 | bevicizumab, ROP
The media loves a good story and when a medication that has been given to children may have a link to “brain damage” you better believe it will gain some traction.
Ever since the first reports began emerging suggesting laser treatment for ROP may have an alternative in Avastin (bevicizumab) skeptics have abounded. After the link was established between high vascular endothelial growth factor (VEGF) and ROP it was not long before the thought of using Bevicizumab to reduce abnormal vessel proliferation of the developing retina. This medication is an anti-VEGF medication that had been used for treatment of colorectal cancer and with this link to ROP a new use had been found for an existing drug. It was big news and created a great stir in many units around the world.
What followed was solid research demonstrating efficacy with the BEAT-ROP being the most influential trial. The full text of this study can be found here. The results of this study of 150 infants (300 eyes) clearly demonstrated that for those with 3+ disease in zone 1, outcomes were better than if laser was used for treatment. For Zone 2 disease there really was no benefit to Bevicizumab but due to the ablative nature of laser, infants in the long run (particularly for Zone 1 disease) can be left with visual compromise later in life including myopia, strabismus as well as amblyopia.
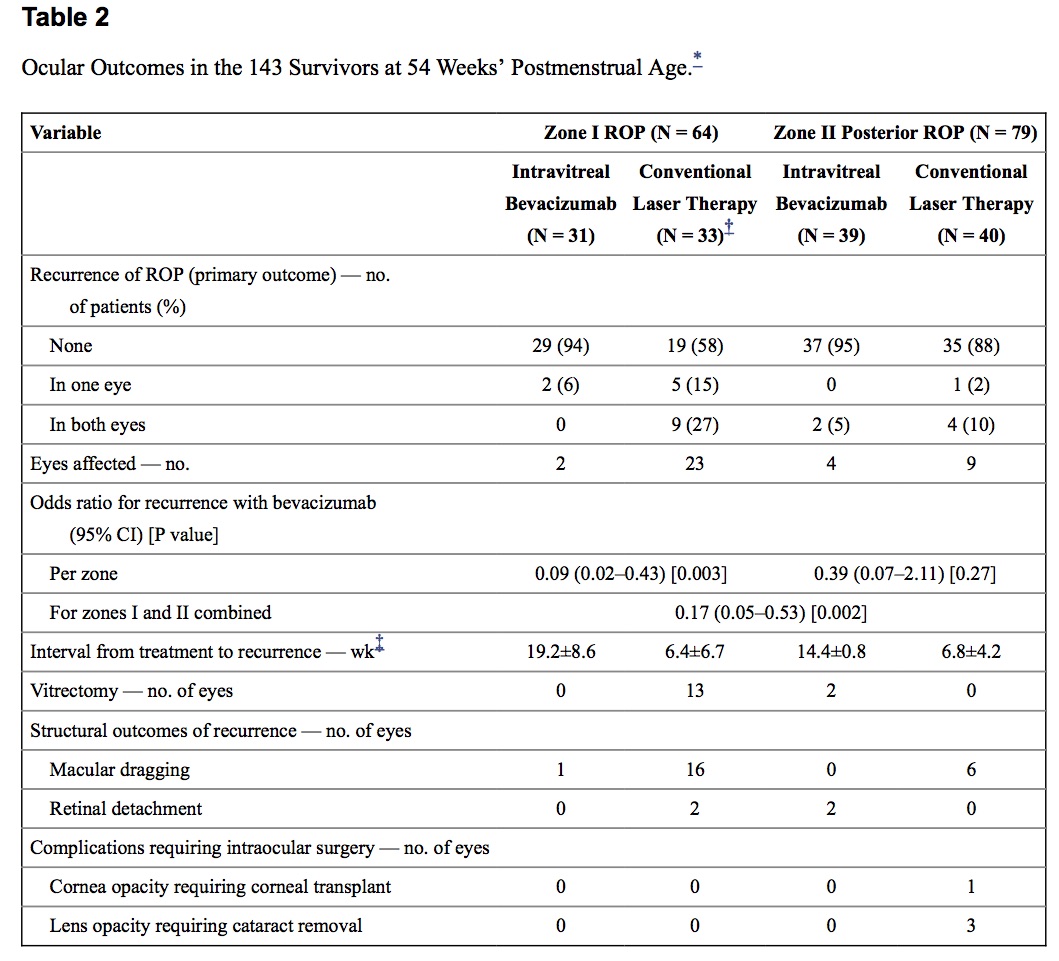
It is for these reasons that I have to confess I have been quite keen on Bevicizumab although at least in our own centre laser in general continues to be presented as standard of care with the option for injection. For Zone 1 disease though Bevcizumab is becoming increasingly recommended due to the aforementioned visual sparing effects in the long run.
What is the concern with Avastin?
The main issue as with any new treatment is the question of whether there are any long term consequences to using a medication that could distribute systemically. We know that many ocular medications can enter the rest of the body and given that VEGF is found in virtually every organ system in the body it is appropriate to express some caution before laser is replaced as standard of care. Anti-VEGF activity could for example cause abnormal vascular development in the lungs of these fragile infants who are trying to recover from BPD and make a disease that already has a vascular component worse. A more direct concern could be the effect of altering vascular development so close to the brain with the potential fear of causing developmental impairment as a result. It may seem surprising that there is so little out there on the topic until you consider that the BEAT-ROP study was published in 2011. This is the time five years later that we should expect to see some outcome results and now we have.
This was the title this week of an AAP update after the following article was published Neurodevelopmental Outcomes Following Bevacizumab Injections for Retinopathy of Prematurity by Morin J using data from the Canadian Neonatal Network. The trial is important as data is needed on the outcome of these infants but the message that is coming out of this publication is worrisome to me in that conclusions are being drawn from a study that has significant concerns. The main findings that have raised many an eyebrow were the following comparing injection to laser in that order for the percentages.
Neurodevelopmental Impairment: 78% vs 56%
Severe Neuro disability: 52% vs 29%
The usual concerns are here in that the study is retrospective and therefore prone to the usual types of error in having groups unbalanced. One of these imbalances is in the severity of illness at admission as measured by the SNAP-II score. The babies treated with injections were sicker and significantly so. The scores in this case were medians of 24 in the Bevicizumab group vs 19 in the laser group. This is quite significant as another group using the CNN data previously published their findings in 2013 that among other things the SNAP-II score was predictive of adverse outcome in a group of infants from 23-30 weeks (same group that is screened for ROP) if the score was above 20. Indeed in the current study the median score for the group treated with Bevicizumab was above this threshold so is the treatment the issue or the fact that the babies were sicker to begin with?
The second significant concern I have with this study is the nature of the babies who were treated with injection vs laser. Based on the BEAT-ROP study most centres reserve injections for the most severe Zone 1 disease and that was no different here. The babies receiving injections had aggressive Zone 1 disease 19% of the time compared to the laser group at 7%. These babies had worse eye disease but how does that tie in to long term neurodevelopment? Several papers have linked adverse developmental outcome by itself to the severity of ROP that the infants had. One such paper by Msall looked at the outcome from the CRYOROP study and found exactly this in a follow up of 1199 survivors so a very large study to draw conclusions from.
So we have the current study suggesting that adverse neurodevelopmental outcome is associated with receiving injections for ROP. We also have two very clear confounders that one cannot ignore. The babies who received injections were both sicker at admission and had worse eye disease overall which both are predictive of adverse outcome. Add to this that another retrospective study that is not much smaller than this found no difference in outcome and I think the conclusions of the current CNN paper are suspect. Interestingly I don’t recall seeing any media attention for this negative study but then that tends to be the way these types of results are handled. Kind of boring so not worth reporting?
Where do we go from here?
What we really need now is a double blind randomized controlled trial to sort this out. It needs to be large enough such that the SNAP-II scores are not significantly different and injections and laser need to truly go head to head. This could be accomplished by following up the BEAT-ROP group or by repeating another trial with the primary outcome being neurodevelopment. In the meantime I would urge everyone to take a breath and not panic. While I confess I have a bias towards Bevicizumab for its vision sparing advantages there could be an impact on development that we cannot ignore. We need to study this well though and that will take some time. Until we do both the scientific community and the lay press need to carefully weigh the merits of releasing studies with significant sources of confounding risks as a little fear can be a strong motivator and potentially deprive infants in the NICU of an important option for treatment.
by All Things Neonatal | Mar 17, 2016 | antibiotics, MAS, Uncategorized
We live in a world at the moment where the public has become increasingly aware of the dangers of antibiotic overuse. Parents are more than ever requesting no erythromycin for the eyes after birth, and even on occasion questioning the need for antibiotics after delivery for the infant with risk factors for sepsis. The media has latched on to the debate as well by publishing the sensational articles about superbugs and medicine running out of the last lines of defence such as this article from the CBC.
As teams caring for newborns both preterm and term we are also increasingly aware of the dangers of altering the microbiome of these vulnerable infants with antibiotic overuse. Some babies robbed of the vaginal microbiome when delivery occurs by C-section, have their parents swabbing their newborn with vaginal secretions to populate their child with the “good bacteria” that come through a “natural” delivery although recent commentary questions the safety of such practice.
Infants born through meconium stained amniotic fluid can certainly become sick after delivery. Inhalation of meconium in the sickest infants often occurs during gasping episodes in utero after hypoxic stress causes evacuation of the rectal contents. The fetuses who inhale this material may go on to develop, inflammatory changes, areas of atelectasis and hyperinflation and pulmonary hypertension; the so called meconium aspiration syndrome. These infants of course may be extremely sick and need high frequency ventilation to manage their CO2 retention and in some cases may go on to ECMO although with inhaled nitric oxide this has become less common. As another consideration, could infection such as chorioamnionitis be the inciting event to cause passage of meconium in utero?
The health care team though for as long as I have been in practice would add to the treatment plan a course of antibiotics. In fact I would guess that many Neonatologists the world over have uttered the phrase “They are REALLY sick, please start antibiotics”. The real question though is whether the baby is in fact infected. Meconium is certainly a good growth medium for bacteria but with the short time from passage to delivery in most cases I doubt there is much time for significant growth. Moreover, I have found myself saying many times that such infants have a chemical pneumonitis and have often questioned whether antibiotics are really needed. Nonetheless it would take nerves of steel in some cases to not use antibiotics in these patients.
Then along came this study
Role of prophylactic antibiotics in neonates born through meconium-stained amniotic fluid (MSAF)—a randomized controlled trial by Goel A et al. This study was done prospectively by randomizing newborns born through meconium stained amniotic fluid to either antibiotics (N=121) for three days or no antibiotics (N=129) after diagnosis. In each case blood and CRP were drawn and if the infant was symptomatic (presence of respiratory distress, lethargy, abdominal distension, temperature or hemodynamic instability, hypoglycemia, apnea, or any other systemic abnormalities) a lumbar puncture and chest x-ray were added. The primary outcome variable was defined as ” the incidence of early (within first 72 h of birth) or late onset (after 72 h of birth) suspect sepsis (clinical symptoms or positive sepsis screen defined as ≥2 positive parameters) and confirmed sepsis (positive blood culture).”
Results
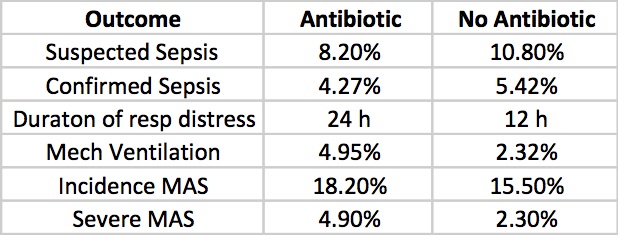
Clinicians in the study were allowed to continue antibiotics past the 72 hours or start antibiotics in the no antibiotic group if they considered an infant to have suspected sepsis or in fact were found to be proven. The outcomes for those possibilities are shown below.
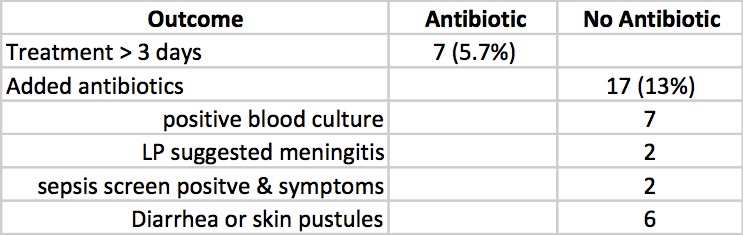
Taking it all together whether you started antibiotics or not the primary outcomes were no different. Furthermore there is no apparent harm based on outcomes that matter including the most important; death (3 in each group) that it does give one reason to pause when considering whether to treat prophylactically with antibiotics for babies born through meconium stained fluid.
What About The Sickest of The Sick
When attempting to answer this question the authors noted the following.
“On doing a subgroup analysis on incidence of sepsis in symptomatic babies (presenting with respiratory distress), both groups were found to have comparable incidence of suspect sepsis (p=0.084). The incidence of confirmed sepsis was more in symptomatic babies, although the total numbers was very few (p=0.01)”
Herein lies the challenge in declaring once and for all that we don’t need antibiotics at all in MAS. While the study was powered to adequately answer the primary outcome, the subgroups are so small that declaring with any confidence that one can stand by and watch infants with severe MAS without starting antibiotics is a tough conclusion to come to. The child though who is born through MSAF and has mild tachypnea as the only symptom I suspect is another story. I might even argue that the baby who is in need of CPAP could be watched and if they deteriorate have antibiotics started. As much as I would love to say none of these babies need antibiotics I would have to admit that I would cave once the baby was ventilated. It is better to provide a couple of days of antibiotics while awaiting blood cultures than to have a patient with sepsis left untreated or at least that is my opinion.
The question is what would you do?
by All Things Neonatal | Mar 9, 2016 | Neonatal, Neonatology, outcome, steroids, Uncategorized
It seems like a sensational title I know but it may not be as far fetched as you may think. The pendulum certainly has swung from the days of liberal post natal dexamethasone use in the 1990s to the near banishment of them from the clinical armamentarium after Keith Barrington published an article entitled The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs in BMC Pediatrics in 2011. This article heralded in the steroid free epoch of the first decade of the new millennium, as anyone caring for preterm infants became fearful of causing lifelong harm from steroid exposure.  Like any scare though, with time fear subsides and people begin asking questions such as; was it the type of steroid, the dose, the duration or the type of patient that put the child at risk of adverse development? Moreover, when death from respiratory failure is the competing outcome it became difficult to look a parent in the eye when their child was dying and say “no there is nothing more we can do” when steroids were still out there.
Like any scare though, with time fear subsides and people begin asking questions such as; was it the type of steroid, the dose, the duration or the type of patient that put the child at risk of adverse development? Moreover, when death from respiratory failure is the competing outcome it became difficult to look a parent in the eye when their child was dying and say “no there is nothing more we can do” when steroids were still out there.
Over the last decade or so, these questions in part have been studied in at least two important ways. The first was to ask whether we use a lower dose of dexamethasone for a shorter period to improve pulmonary outcomes without adverse neurodevelopment? The target population here were babies on their way to developing chronic lung disease as they were ventilated at a week of age. The main study to answer this question was the DART study. This study used a very low total dose of 8.9 mg/kg of dexamethasone given over ten days. While the study was stopped due to poor recruitment (it was surely difficult to recruit after the 2001 moratorium on steroids) they did show a benefit towards early extubation. This was followed up at 2 years with no difference in neurodevelopmental outcomes. Having said that the study was underpowered to detect any difference so while reassuring it did not prove lack of harm. Given the lack of evidence showing absolute safety practitioners have continued to use post natal steroids judiciously.
The second strategy was to determine whether one could take a prophylactic approach by providing hydrocortisone to preterm infants starting within the first 24 hours to prevent the development of CLD. The best study to examine this was by Kristi Watterberg in 2004 Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Strangely enough the same issue of early stoppage affected this study as an increased rate of spontaneous gastrointestinal perforation was noted leading to early closure. The most likely explanation is thought to be the combination of hydrocortisone and indomethacin prophylaxis which some centres were using at the same time. An interesting finding though was that in a subgroup analysis, infants with chorioamnionitis who received hydrocortisone had less incidence of chronic lung disease. (more on this later) Although this of course is subject to the possible bias of digging too deep with secondary analyses there is biologic plausibility here as hydrocortisone could indeed reduce the inflammatory cascade that would no doubt be present with such infants exposed to chorioamnionitis in utero.
Has the answer finally come?
The DART study at 360 patients was the largest study to date to look at prophylaxis as a strategy. That is until this past week. The results of the PREMILOC study have been published which is the long awaited trial examining a total dose of 8.5 mg/kg of hydrocortisone over 10 days. We can finally see the results of a trial without the complicating prophylactic indomethacin trials interfering with results. Surprisingly this study was also stopped early (a curse of such trials?!) due to financial reasons this time. Prior to stoppage though they managed to recruit 255 to hydrocortisone and 266 to control groups. All infants in this study were started on hydrocortisone within 24 hours of age and the primary outcome in this case was survival without BPD at 36 weeks of age.
All infants were less than 28 weeks at birth and therefore had a high risk of the combined outcome and despite the study being stopped early there was indeed a better outcome rate in the hydrocortisone group (60% vs 51%). Another way of looking at this is that to gain one more patient who survived without BPD you needed to treat 12 which is not bad at all. What is additionally interesting are some of the findings in the secondary analyses.
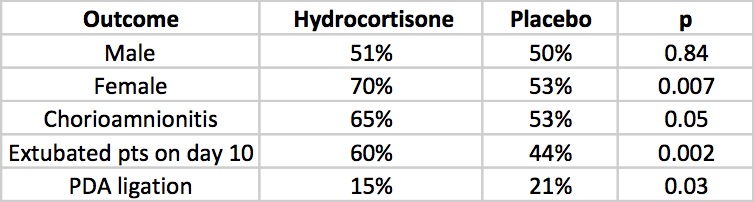
The lack of a difference in males may well reflect the biologic disadvantage that us males face overcoming any benefit from the hydrocortisone. In fact for the females studied the number needed to treat improves to 6 patients only! Short term outcomes of less ventilation are sure to please everyone especially parents. Lastly, a reduction in PDA ligation is most probably related to an antiprostaglandin effect of steroids and should be cause for joy all around. Lastly, a tip of the hat to Dr. Watterberg is in order as those infants who were exposed to chorioamnionitis once again show that this is where the real benefit may be.
But what about side effects?
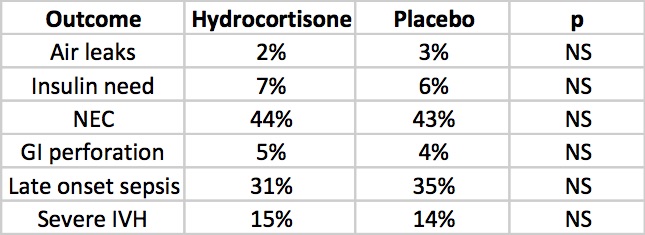
The rate of NEC is quite high but is so for both groups but otherwise there is nothing much here to worry the reader. Once and for all we also see that by excluding concurrent treatment with indomethacin or ibuprofen the rate of GI perforation is no different this time around. Reassuring results indeed, but alas the big side effect, the one that would tip the scale towards us using or abandoning treatment has yet to be presented. Steroids no doubt can do great things but given the scare from 2001 we will need to see how this cohort of babies fares in the long run.
The follow-up is planned for these infants and the authors have done an incredible job of recruiting enough patients to make the results likely believable. I for one can’t wait to see what the future holds. If I was a betting man though I would say this ultra low dose of hydrocortisone may be just the thing to bring this therapy finally into the toolbox of neonatal units worldwide. We have been looking for the next big thing to help improve outcomes and good old hydrocortisone may be just what the doctor ordered.

 The 3 dose schedule was used for those infants born between 29 0/7 and 35 weeks gestational age who had a risk factor score of 42 or more. Interestingly at the end of the RSV season, depriving such infants of 1 or 2 doses did not appear to impair the ability of the infant to maintain protective levels.
The 3 dose schedule was used for those infants born between 29 0/7 and 35 weeks gestational age who had a risk factor score of 42 or more. Interestingly at the end of the RSV season, depriving such infants of 1 or 2 doses did not appear to impair the ability of the infant to maintain protective levels.







 Like any scare though, with time fear subsides and people begin asking questions such as; was it the type of steroid, the dose, the duration or the type of patient that put the child at risk of adverse development? Moreover, when death from respiratory failure is the competing outcome it became difficult to look a parent in the eye when their child was dying and say “no there is nothing more we can do” when steroids were still out there.
Like any scare though, with time fear subsides and people begin asking questions such as; was it the type of steroid, the dose, the duration or the type of patient that put the child at risk of adverse development? Moreover, when death from respiratory failure is the competing outcome it became difficult to look a parent in the eye when their child was dying and say “no there is nothing more we can do” when steroids were still out there.
