by All Things Neonatal | Oct 8, 2015 | antibiotics
The literature over the last few years has ballooned with reports of adverse outcomes related to antibiotic overuse. The most common adverse outcome noted on the worldwide stage is that of antimicrobial resistance and the so-called “superbugs” but in the NICU a whole host of other outcomes have been noted. Prolonging antibiotics beyond the first couple days of life has led to changes in the microbiome towards more pathogenic bacteria in the NICU. This shift has been accompanied by a rise in the rate of necrotizing enterocolitis in such units as well as many outcomes including some which affect us as adults such as asthma, which has been discussed on my Facebook page before.
New Consequences of Prolonging Antibiotics Beyond 48 hours
This month a colleague with a particular interest in this story forwarded our team the following paper. Prolonged Early Antibiotic Use and Bronchopulmonary Dysplasia in Very Low Birth Weight Infants by Novitsky et al. What this retrospective study demonstrated (comparing 747 infants treated for less than 48 hours to 159 treated beyond) was that the prolongation of antibiotics beyond the first 48 hours was associated with increased propensity to develop BPD. This finding remained true even when factoring in other risks for such outcomes including gestational age, maternal antibiotics, inborn status, clinical chorioamnionitis, prolonged rupture of membranes, preeclampsia, cesarean delivery, Apgar at 5 minutes, SNAP score, and mechanical ventilation. Curiously it is not just the initial course that is of concern but ongoing exposure as well. “After adding total cumulative hospital course antibiotic days, the odds of BPD remained increased with > 48 hours of antibiotic coverage in the 1st week. Furthermore, for every additional day of antibiotic coverage after the 1st week of life, there was an increased odds of developing BPD (OR, 1.16 per antibiotic day, 95% CI, 1.11–1.2). “
Prolonging antibiotics has another downstream consequence of increasing the rates of endotracheal tube colonization with bacteria to the tune of 38 vs. 16%. Additionally there was a shift in endotracheal tube culture patterns towards the emergence of resistant gram negative organisms (7% vs. 2%) if prolonged treatment up to 7 days was chosen. As has been shown in other studies rates of NEC were increased as well which should be no surprise based on previous similar work.
Wanting To Be Safe And Do The Right Thing
Once bitten twice shy as the saying goes. Any clinician who has missed an episode of sepsis in a newborn after not starting antibiotics is no doubt scarred to some degree. I have no doubt that all of us wish to do the right thing and protect the infants in our care when risk factors for sepsis indicate to us that there is a higher likelihood of sepsis being present. Looking at this study one can see the following risks were indeed more prevalent in the group treated beyond 48 hours.
| Risk |
Antibiotics < 48 hours in 1st week |
Antibiotics > 48 hours in 1st week |
P value |
| Birth weight |
1053 +/- 296 |
944 +/- 274 |
<0.01 |
| SNAP score |
13 +/- 9.2 |
17.8 +/-7.1 |
<0.01 |
| Clinical Chorio % |
5 |
18 |
<0.01 |
| Prolonged ROM |
14 |
26 |
<0.01 |
From the table I think we can agree that these infants were smaller and potentially sicker which likely motivated those clinicians to try and “do the right thing” even if cultures were negative. In this paper they defined sepsis as a positive culture and the presence of signs suggestive of sepsis, which avoids any confusion, related to such subjective classification as “possible or probable” sepsis where the clinical picture is suggestive but the culture negative. Despite a perceived increase in risk of sepsis after birth how accurate were clinicians? Early and Late Onset Sepsis Rates 14% in the < 48 hr and 16% in the > 48 hour group. Not significant at all.
The Cost of Fear
What is significant is the consequence of fear that motivates such decisions in the face of negative blood cultures. There is no difference in our ability to predict, yet doing so increases the risk of recovering bacteria from the endotracheal tube and more so resistant ones. The likelihood of obtaining bacterial growth in the endotracheal tube is more than doubled; NEC increased 2.5 times, gram-negative resistance tripled and the odds of developing BPD approximately doubled with an increasing tendency to this outcome simply by prolonging antibiotics. The cost of fear is that we trade our poor ability to truly predict sepsis with significant adverse risk that impacts the infant not only during their stay but long after they leave the NICU.
It is human nature to wish to do no harm. In order to truly achieve this we have to quell our need to “feel good” by knowing we have covered for sepsis and replace this with the fear of causing serious harm from such action. The decision to prolong the antibiotic course is the easy route as we go home comfortable that the baby is “covered” in case we are wrong. It makes us feel better but at significant cost. Only by changing our perspective of what constitutes harm will we ever move forward in this fight to change practice. Are you prepared to do it?

by All Things Neonatal | Oct 5, 2015 | Neonatology, preemie
* I would like to thank Jennifer Degl for providing permission to use her photo for this post. She is the author of From Hope to Joy and does great work which can be found at: www.micropreemie.net
A publication this past week has been featured in multiple news stories across North America due to it’s impact on mortality and morbidity in the NICU. Shielding Parenteral Nutrition From Light Improves Survival Rate in Premature Infants: A Meta Analysis made the splash that it did because it’s premise is so simple yet has such an impact. In essence, protect TPN from light (including phototherapy) and you can cut mortality in the NICU in half!
A Canadian Research Story
The CBC has covered this as well with the following piece that also indicates that a survey of NICUs from 4 years ago indicated about half of hospitals did not employ such shielding. In fairness the meta-analysis has just been published which combined 4 studies and about 800 patients to yield these findings but the understanding that such practice could benefit newborns in NICU has been known for many years. What makes this story even more interesting to me is it’s Canadian origin in that Dr. Chessex performed much of the work in this field and his dedication to the area of oxidative stress in large part led to this finding.
In fact in 1999 he published the following paper Protecting solutions of parenteral nutrition from peroxidation which demonstrated that simply covering the bag of TPN was not enough to prevent oxidation from occurring. The whole set up including the bag, lines and during the preparation of TPN needed to be shielded or peroxide concentrations increased by 1.5 -2 times compared to when a clear set up was used. Furthermore phototherapy led to a further rise in the concentration of these oxidative harmful molecules. Ironically it is the necessary components of TPN including riboflavin and lipid that create the environment for light to create these oxidative products that can damage tissue.
You may ask yourself at this point why something that was known nearly 17 years ago did not lead to widespread adoption by NICUs across Canada and perhaps North America. For one, medicine is notoriously slow to change practice especially when there is an effort and cost that will need to be considered. Sourcing such materials is actually more difficult than it may seem as we learned locally two years ago when one of our hospitals began this change. Secondly, Neonatology is littered with bench research that while striking in its findings simply did not translate into a clinically relevant outcome. For example we know that phenobarbital increases the conjugation of bilirubin in the liver and therefore in theory should be a great adjunctive treatment to phototherapy for the usual newborn jaundice but that didn’t pan out in human trials. What is the story here though?
The Landmark Study Results That Made Headlines
The meta-analysis mentioned in the start of this piece and causing all this attention included four studies that examined possible reductions in mortality. In 2007 Chessex studied the effect of light protection (LP) on the incidence of BPD finding a 30% reduction in those infants in a randomized study of LP vs none. This finding alone should be enough to raise some eyebrows and it did as many centres were adopting LP around this time. The second study done in Egypt in 2009 demonstrated a similar finding in reduced BPD rates. The third study was by Chessex again in 2009 and once more demonstrated reductions in oxidant stress and BPD. Curiously the largest of the studies based out of France with 587 patients in 2014 randomized to LP or none found no difference in BPD or death but the latter was very close to meeting statistical significance. In all of these studies no difference in mortality was noted however when they were combined and examined as a group the following was identified. 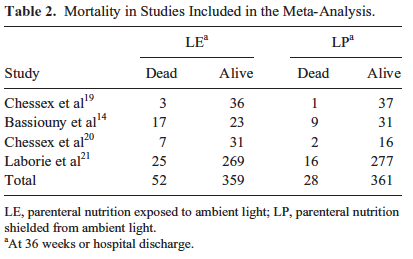 Herein lies the power so to speak of the meta-analysis. Small studies may not demonstrate a difference that reaches significance in the desired outcome of interest but if several studies that have very similar measurements are pooled together the power to find a difference may emerge. That would seem to be the case here in that not only is there a halving of mortality that reaches statistical significance but a specific disadvantage for males was uncovered in that they had a two fold risk compared to females of dying if their TPN was uncovered.
Herein lies the power so to speak of the meta-analysis. Small studies may not demonstrate a difference that reaches significance in the desired outcome of interest but if several studies that have very similar measurements are pooled together the power to find a difference may emerge. That would seem to be the case here in that not only is there a halving of mortality that reaches statistical significance but a specific disadvantage for males was uncovered in that they had a two fold risk compared to females of dying if their TPN was uncovered.
Are These Results Relevant to Modern Practice
The results of this study are profound in terms of the impact that they could have on both BPD and mortality in our NICUs. One caveat needs to be mentioned however and that is the utlilization of oxygen in the NICU now and during the time of these studies. Since the time periods that these studies were undertaken, the use of oxygen for many units including our own has become more tightly regulated. As per NRP guidelines we resuscitate our newborns with room air and use every effort at the bedside to avoid wide swings of FiO2 when addressing an episode of desaturation. Furthermore, antenatal steroids, surfactant, liberal use of CPAP have all led to marked reductions in need for FiO2 such that the days of infants being on 30% FiO2 by nasal prongs have been replaced by room air on CPAP for the most part (at least in our units). What would these results be now if these studies were repeated? My suspicion is not as dramatic but there is no question that for at least 17 years we have known about the risk of such oxidative stress.
Is there any logic behind waiting for more evidence in a modern cohort before implementing a strategy of protecting these solutions from light? I don’t think so and hope that the rest of our community agrees and does not wait many more years to implement such precautions. How many other conditions such as ROP could be affected by simply protecting these solutions from light? Quite frankly I don’t need to know. The time has come for change.
Thank you Dr. Chessex for your dedication in bringing this translational research from the bench to the bedside.

by All Things Neonatal | Oct 1, 2015 | Prematurity
Its hard not to hear about probiotics these days. They are in our grocery aisles as supplements to yoghurt and other foods and can be purchased in health food stores or at your local pharmacy. They appear to be everywhere as word spreads about the importance of your microbiome in maintaining good overall health. The first food to have probiotics added to it was a Japanese drink called Yakult  and after that the concept took off with Danone’s Activa drink not being far behind. Medicine has studied the effects of probiotics for all sorts of conditions with a pubmed search for reviews of probiotics in humans coming up with over 1800 results. The concept is enticing for sure. Take bacteria which are known to populate healthy intestines and provide them either in food or in capsules to change the bacterial population in patients who for one reason or another have cause to believe their intestinal microbiome has shifted to something undesirable.
and after that the concept took off with Danone’s Activa drink not being far behind. Medicine has studied the effects of probiotics for all sorts of conditions with a pubmed search for reviews of probiotics in humans coming up with over 1800 results. The concept is enticing for sure. Take bacteria which are known to populate healthy intestines and provide them either in food or in capsules to change the bacterial population in patients who for one reason or another have cause to believe their intestinal microbiome has shifted to something undesirable.
Do Probiotics Reduce The Risk of Necrotizing Enterocolitis?
It didn’t take long for clinician scientists to turn their attention to the neonate who is at risk of necrotizing enterocolits (NEC). It has been known for some time that formula feeding versus breast milk plays a role in the development of NEC as premature infants fed formula repeatedly were found across studies to have a higher incidence of NEC. The evidence is so strong in fact that the Cochrane review on the subject states “Enteral supplementation of probiotics prevents severe NEC and all cause mortality in preterm infants. Our updated review of available evidence strongly supports a change in practice.” If you have read such reviews you know that they rarely come out this strong in their support of something! Furthermore, infants fed formula may have a different preponderance of more pathogenic bacteria in the colon and less lactobacillus and bifidobacterium species. The idea behind providing probiotics to neonates would therefore be to repopulate the intestine of these vulnerable infants with good bacteria and potentially reduce the incidence of a devastating condition like NEC.
If only it were that easy though. The issue of using Probiotics in preterm infants is a contentious one to say the least. While the evidence appears to indicate an overall benefit in terms of reducing rates of NEC there remain concerns regarding the safety of providing bacteria to this population even though the bacteria are thought to be beneficial. The people who urge caution in the use of probiotics say so due to a few reports of sepsis after the introduction of probiotics with the organism that the patient was provided or with a different species that was could be traced to a contaminated product. In fact the contamination need not be with bacteria as was found in 2014 when Rhizopus oryzae was found in a commonly used product as was described in the Forbes article here. What made this case garner widespread media attention was that the infant died and justifiably led people to start asking questions about safety.
How Certain Are We of The Quality of the Products?
As I mentioned in another post on Facebook and Twitter recently, these products fall under the category of a nutritional product rather than a medication with Health Canada and therefore are not subject to the same rigorous quality control standards as other comparable medications would be.
An additional challenge (emanating from the lack of rigorous quality control demanded by a product deemed a drug) is the certainty that what you believe you are administering is actually in the product on the label. I demonstrated above how there can be contaminants but shouldn’t the product actually have at least the bacteria that is on the bottle as well? The majority of probiotics destined for human consumption are meant to contain lactobacillus and/or bifidobacterium. The question however is whether they actually do when independent analysis is performed. Sadly independent studies have confirmed that this may not be the case. A 2004 study by Szajewska and colleagues found that only 3/5 products tests contained the bacteria that were labelled on the bottle. Similarly Toscano et al in 2013 examined products in the European market to determine quality. Interestingly a decade later their findings were the same in that 58% of 24 products contained what they claimed. Clearly just because it says it contains these bacteria on the bottle doesn’t necessarily mean that.
Hard To Argue About Sepsis?
Taking all of this theoretical concern into consideration, one has to acknowledge an additional and very important finding from a
recent systematic review. In a pooled analysis of 25 studies on the use of probiotics the risk of sepsis was reduced in the following manner:
1. risk of any sepsis (25 RCTs; RR 0.83, 95% CI 0.73-0.94; I = 26%)
2. bacterial sepsis (11 RCTs; RR 0.82, 95% CI 0.71-0.95; I = 0%),
3. fungal sepsis (6 RCTs; RR 0.57, 95% CI 0.41-0.78; I = 0%)
4. This beneficial effect remains in very low birth weight infants (<1500 g) (19 RCTs; RR 0.86, 95% CI 0.75-0.97; I = 18%), but not in extremely low birth weight infants (<1000 g) (3 RCTs; RR 0.73, 95% CI 0.45-1.19; I = 53%).
A reduction in sepsis is not anything to take lightly. Late onset sepsis in the NICU can be very damaging and may predict poorer development at 18 months and beyond. In this study, despite the aforementioned concerns regarding potential infection with the organism provided no such infections occurred.
Despite all the possible benefits, in the NICU much attention has moved towards donor milk and moreover establishing exclusive human milk diets. With this changing landscape the beneficial effects of probiotics may decrease over time. Given that the goal of probiotics is to change the microbiome to one that is closer to a breastfed infant will this still be needed? What we will need for sure is a repeating of trials in units that have high rates of human milk exposure to know for sure.
Whether to use probiotics or not becomes a tough question to answer and would be primarily based on your sense of trust in the quality control of the products at the moment I would think. The benefits appear to be real but the question is, are you willing to take the risk?



 Herein lies the power so to speak of the meta-analysis. Small studies may not demonstrate a difference that reaches significance in the desired outcome of interest but if several studies that have very similar measurements are pooled together the power to find a difference may emerge. That would seem to be the case here in that not only is there a halving of mortality that reaches statistical significance but a specific disadvantage for males was uncovered in that they had a two fold risk compared to females of dying if their TPN was uncovered.
Herein lies the power so to speak of the meta-analysis. Small studies may not demonstrate a difference that reaches significance in the desired outcome of interest but if several studies that have very similar measurements are pooled together the power to find a difference may emerge. That would seem to be the case here in that not only is there a halving of mortality that reaches statistical significance but a specific disadvantage for males was uncovered in that they had a two fold risk compared to females of dying if their TPN was uncovered.
